Rivenin
Well-Known Member
- Joined
- Dec 13, 2010
- Messages
- 3,258
- Reaction score
- 342
SO, i've been having this issue with JUST my IPAs.
Everything else turns out fine, stout, porter, pale mild, sours, etc. And it's just a recent occurrence. I've brewed some amazing IPAs and IIPAs in the past, but since i moved back into portland, i decided to give the tap water a shot.
Long story short, there is barely any hop flavor or smell at all, it just smells and tastes like a weird caramel, not diacetyl, not really off, just, it has no hops. And the same exact flavor has been there in all my hop forward beers recently.
Pulled a water report and we are pretty much close to 0 on everything and we also have chloramines... so i use campden.
While we are close to 0, i use the water chemistry primer as well to add salts and some acid malts since i started using tap water, all my IPAs i've made have pretty much been a waste of money, which isn't great because funds are limited for me . So i have a feeling the water run offs changed, so something happened with the water recently... but i don't want to waste money anymore on my expensive hoppy beers and would rather have a plan in place. Also, i live in outer south east, so i may not always be on the same portland water.
. So i have a feeling the water run offs changed, so something happened with the water recently... but i don't want to waste money anymore on my expensive hoppy beers and would rather have a plan in place. Also, i live in outer south east, so i may not always be on the same portland water.
Now, in the past, i've always brought my 5 gallon jugs to the store, filled them up and brewed with spring water... i just got tired of lugging the 10 gallons of water to and fro.
So, i think i'm going to bite the small bullet and start buying spring water again (it's like $3 a batch, so it's not a huge ordeal). But i'm going to brew 2 twin recipes to make sure the water is my culprit (i'm pretty sure it is, but i'd rather run some tests to be positive!)
Now, i want something to turn around semi quick, but i also want something hop forward to see if and where it dulls out. and since i have a few extra oz of galaxy, this is the plan. (i have some mosaic too, but trying to save that for an american wheat, but can swap them if mosaic might be the better choice for this kind of test?)
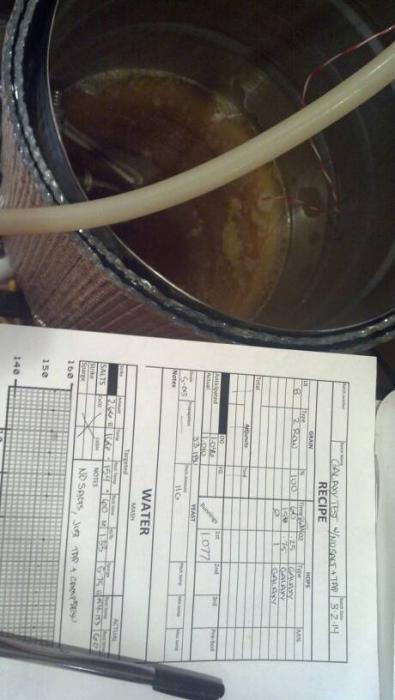
8lb maris otter
.25 galaxy @60
.75 galaxy @15
1 galaxy @0
S-05
mash at 155*
should be 1.046 and 33IBU
1 with plain tap water (treated with campden only)
1 with spring water from the store
going to ferment it out for 1 week in my fermentation fridge, 1 week in my closet (To make space in the fridge, it stays around 65) and brew these 1 week apart. Keg for 2 weeks and do a side by side. (1 month for a 1.046 beer seems like plenty)
Anyone see any hiccups i'm missing here?
Everything else turns out fine, stout, porter, pale mild, sours, etc. And it's just a recent occurrence. I've brewed some amazing IPAs and IIPAs in the past, but since i moved back into portland, i decided to give the tap water a shot.
Long story short, there is barely any hop flavor or smell at all, it just smells and tastes like a weird caramel, not diacetyl, not really off, just, it has no hops. And the same exact flavor has been there in all my hop forward beers recently.
Pulled a water report and we are pretty much close to 0 on everything and we also have chloramines... so i use campden.
While we are close to 0, i use the water chemistry primer as well to add salts and some acid malts since i started using tap water, all my IPAs i've made have pretty much been a waste of money, which isn't great because funds are limited for me
Now, in the past, i've always brought my 5 gallon jugs to the store, filled them up and brewed with spring water... i just got tired of lugging the 10 gallons of water to and fro.
So, i think i'm going to bite the small bullet and start buying spring water again (it's like $3 a batch, so it's not a huge ordeal). But i'm going to brew 2 twin recipes to make sure the water is my culprit (i'm pretty sure it is, but i'd rather run some tests to be positive!)
Now, i want something to turn around semi quick, but i also want something hop forward to see if and where it dulls out. and since i have a few extra oz of galaxy, this is the plan. (i have some mosaic too, but trying to save that for an american wheat, but can swap them if mosaic might be the better choice for this kind of test?)
8lb maris otter
.25 galaxy @60
.75 galaxy @15
1 galaxy @0
S-05
mash at 155*
should be 1.046 and 33IBU
1 with plain tap water (treated with campden only)
1 with spring water from the store
going to ferment it out for 1 week in my fermentation fridge, 1 week in my closet (To make space in the fridge, it stays around 65) and brew these 1 week apart. Keg for 2 weeks and do a side by side. (1 month for a 1.046 beer seems like plenty)
Anyone see any hiccups i'm missing here?




















































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)