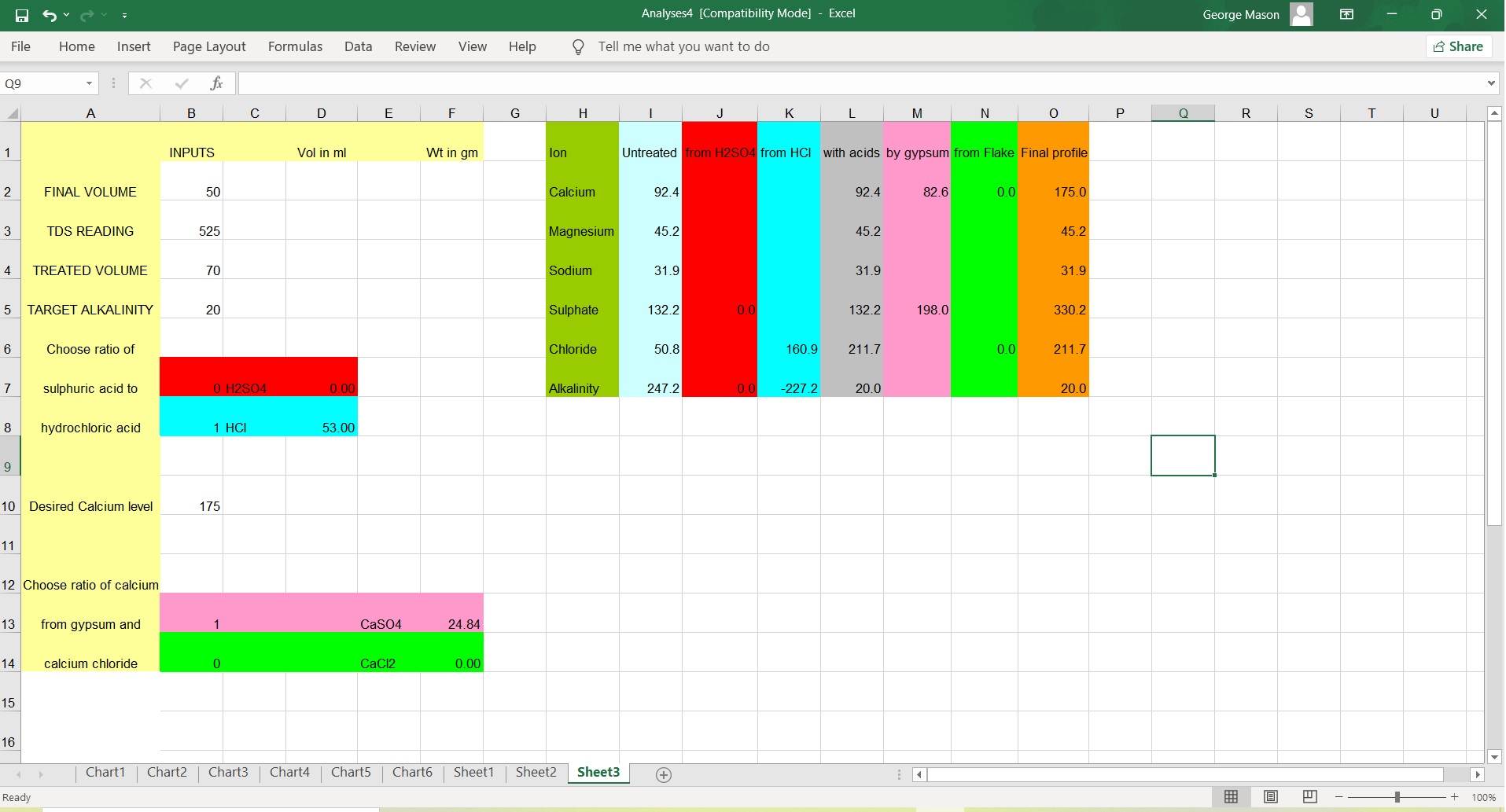
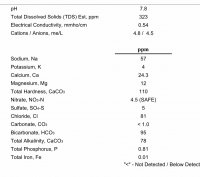
In Basic terms, calcium, magnesium, sodium, sulfate and chloride cannot be added individually to water, those come from compounds we might call salts, acids and bases and there is a fixed and simple numeric ratio of the ions in such compounds. In chemistry the individual ions are electrically charged and know as cations (CA, Mg, Na) and anions (SO4, chloride and carbonate or bicarbonate) and they should balance. A water that does not have equivalence of cations and anions cannot exist and is impossible. The first water profile consisted of 6.99 milliequivalents of cations, with only 4.34 for anions, a significant imbalance. As no amount was given for alkalinity, I assumed this was omitted, but balancing the profile required a substantial quantity of alkalinity, totally unsuited to a Bitter beer or the grist of your recipe.
Now the alternative profile looks better, but again doesn't balance suggesting it would balance if alkalinity was around 80 ppm measured as CaCO3 and while this is less than the first, it is still too much to achieve a reasonable pH in the mash. I know it is common practice in some parts of the globe to mash in, measure pH and throw an acid or a base until the target pH is obtained, but that isn't English brewing practice, where the profile, including alkalinity, is determined beforehand to balance with the chosen grist.
I hope this helps.
Do not add gypsum to a finished beer to assume had gypsum been added before the mash, boil, fermentation the result would be exactly the same. You cannot alter the result of a football game after the final whistle has blown and the same applies to beer. Gypsum and calcium chloride spend several hours then days in chemical and biological reactions resulting in most of the calcium being replaced by potassium and deposited with phosphates and this type of substitution is perhaps the greatest reason for homebrewers the world over misinterpreting the taste of British Cask Ale.








































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)