But still fun....

Two degree's? Congrats! :rockin:
Every good discussion starts with a little degree dropping...
Being an educated individual you should be aware of the mechanism by which hypochlorite results in microbial destruction. As it is pertinent to your above statements, I'ld like to point out some relevant reactions:
1) 2HOCl -> 2HCl + O2
2) HCl + HOCl <-> Cl2 + H2O
Both of these reactions contribute to the microbicidal activity of household bleach: 1) singlet oxygen is a powerful oxidant that will disrupt lipid bilayers and oxidize surface proteins and 2) chlorine can freely diffuse the cell membrane, allowing it to chlorinate proteins within the cell.
Balanced reaction from #1 and #2: 3HOCl -> HCl + O2 + Cl2 + H2O
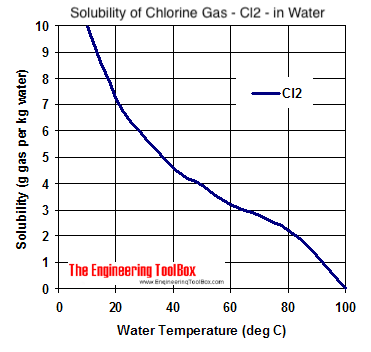
Solubility of chlorine gas: aprox. 7g Cl2/1 L H2O
Reccomended sanitizing concentration of sodium hypochlorite: 1%
Calculate the theoretical yield of Cl2
Assumption: pH <= 5.4 for 99+% formation of HOCl
Solutuion prepared with distilled water
(10 g NaOCl per 1 L H2O) / (77.44 g NaOCl per mole) = 0.129 M NaOCl
0.129M NaOCl @ pH = 5.4 -> 0.129 M concentration of HOCl
1 mole Cl2 / 3 moles HOCl * 0.129 M HOCl = 0.043 M Cl2
0.043 M Cl2 * 70.096 g Cl2 per mole =
3.01 g Cl2 / 1 L H2O
Translation: No chlorine gas will evolved at proper sanitizing concentrations under strongly acidic pH conditions. Or, in other words,
the brewer lives.
Done properly the pH of the hypochlorite solution can be safely adjusted to 7.4 in distilled water to maximize the generation of chlorine and singlet oxygen thereby improving the sanitizing capabilities of the solution. Commercial bleach solutions have a pH = aprox. 12. If your tap water is sufficiently buffered to maintain a pH = 7.4-8.4 after making a 1% bleach solution, there is no reason to add additional acid. However, if the sanitizing solution has a pH > 8.4, reducing the alkalinity of the solution with a dilute weak acid (acetic acid, i.e. vinegar) can improve the effectiveness.



 But still fun....
But still fun....