Holden Caulfield
Well-Known Member
- Joined
- Apr 21, 2020
- Messages
- 318
- Reaction score
- 337
Hey All:
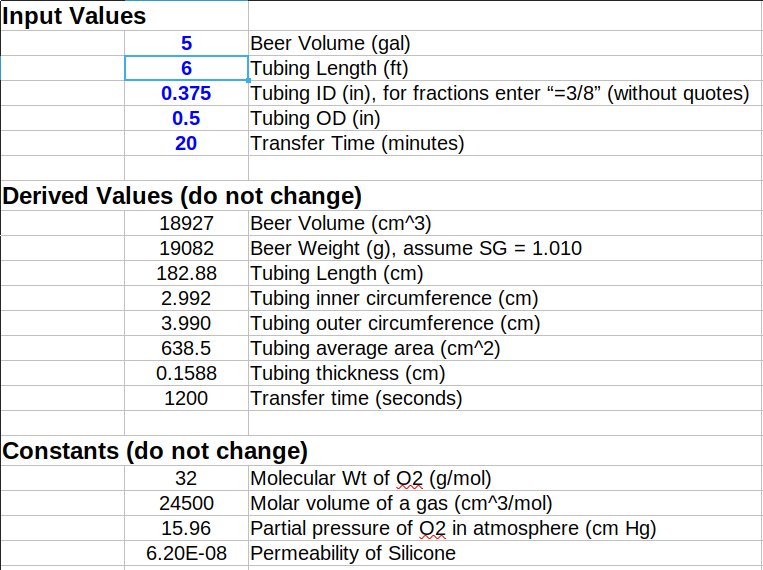
I have been using a 6 foot 3/8" ID silicon tube to close transfer beer from a stainless steel Brewbucket to a CO2 purged keg - for sanitation I boil it prior to using which is why I like it. I know that silicone tubing is notorious for oxygen permeability, but given the short time the racking process takes (about 20 mins), I never gave it a thought. The other day though, I started to think that given the movement of the beer through the tubing and the surface to volume ratio of the tubing to the beer, this could actually be oxidizing my beer during the kegging process. While me beer is very good, especially when comparing to any American Craft Brewer's, when I compare it to German brewers like Tucher, Bitburger, and Ayinger, I am quite humbled.
Any thoughts or better yet, real data, on what I may be doing to my beer during transfer with silicone tubing?
My problem may also be on the hot side as my setup and energy level doesn't really allow for good hot side low oxygen brewing but suffice it to say it pretty standard for a cooler mashtun immersion chiller guy - so not so bad.
Thanks in advance.
I have been using a 6 foot 3/8" ID silicon tube to close transfer beer from a stainless steel Brewbucket to a CO2 purged keg - for sanitation I boil it prior to using which is why I like it. I know that silicone tubing is notorious for oxygen permeability, but given the short time the racking process takes (about 20 mins), I never gave it a thought. The other day though, I started to think that given the movement of the beer through the tubing and the surface to volume ratio of the tubing to the beer, this could actually be oxidizing my beer during the kegging process. While me beer is very good, especially when comparing to any American Craft Brewer's, when I compare it to German brewers like Tucher, Bitburger, and Ayinger, I am quite humbled.
Any thoughts or better yet, real data, on what I may be doing to my beer during transfer with silicone tubing?
My problem may also be on the hot side as my setup and energy level doesn't really allow for good hot side low oxygen brewing but suffice it to say it pretty standard for a cooler mashtun immersion chiller guy - so not so bad.
Thanks in advance.