Think you-all are overcomplicating this. Very little specialized equipment is needed to make a decent agar plate. But, you do need to appreciate that there's a big difference between the sanitation we use in brewing and sterile technique. I'll try to walk you through what I do at home. It's actually very close to what I would do in the lab, back in the day.
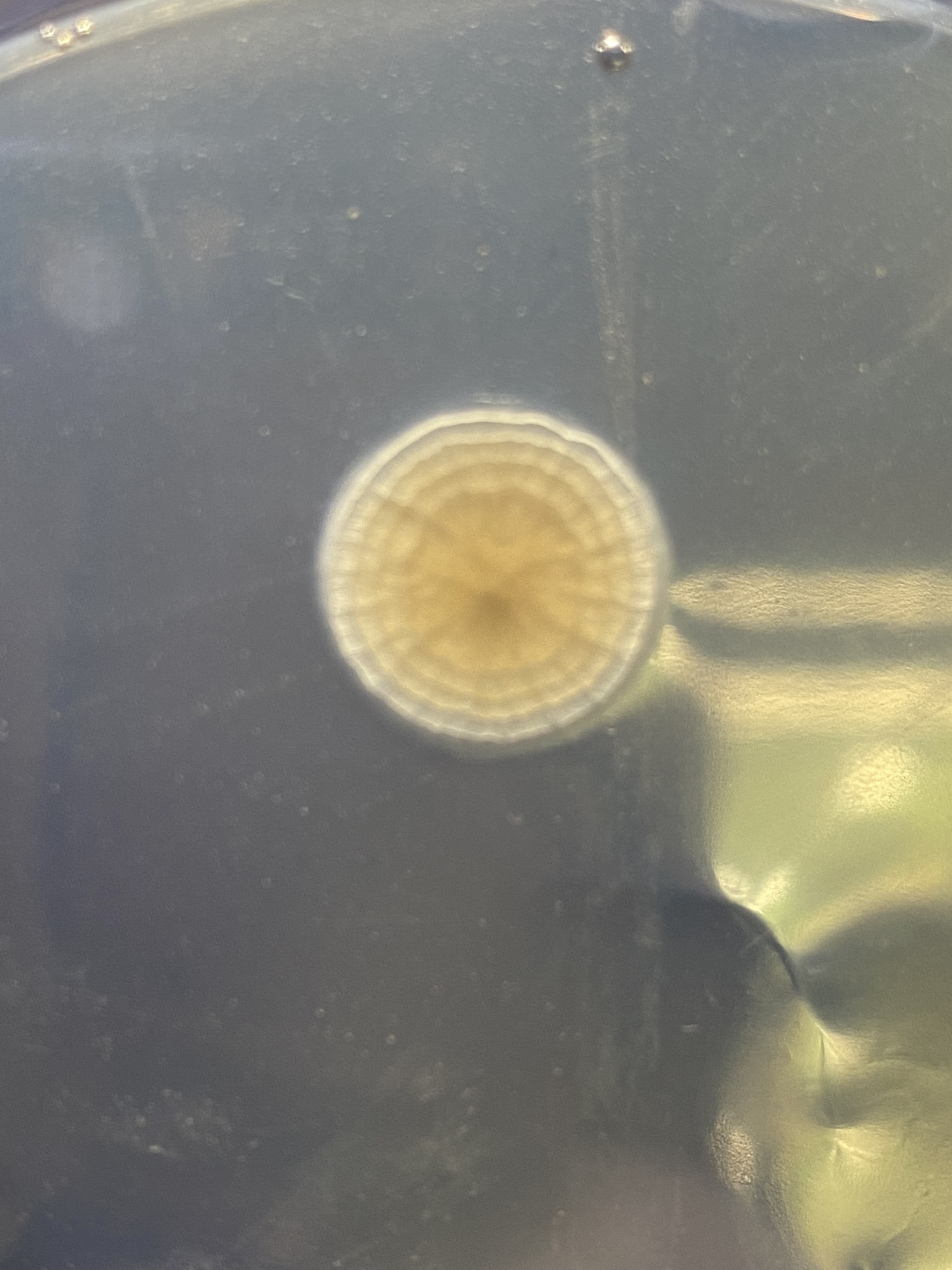
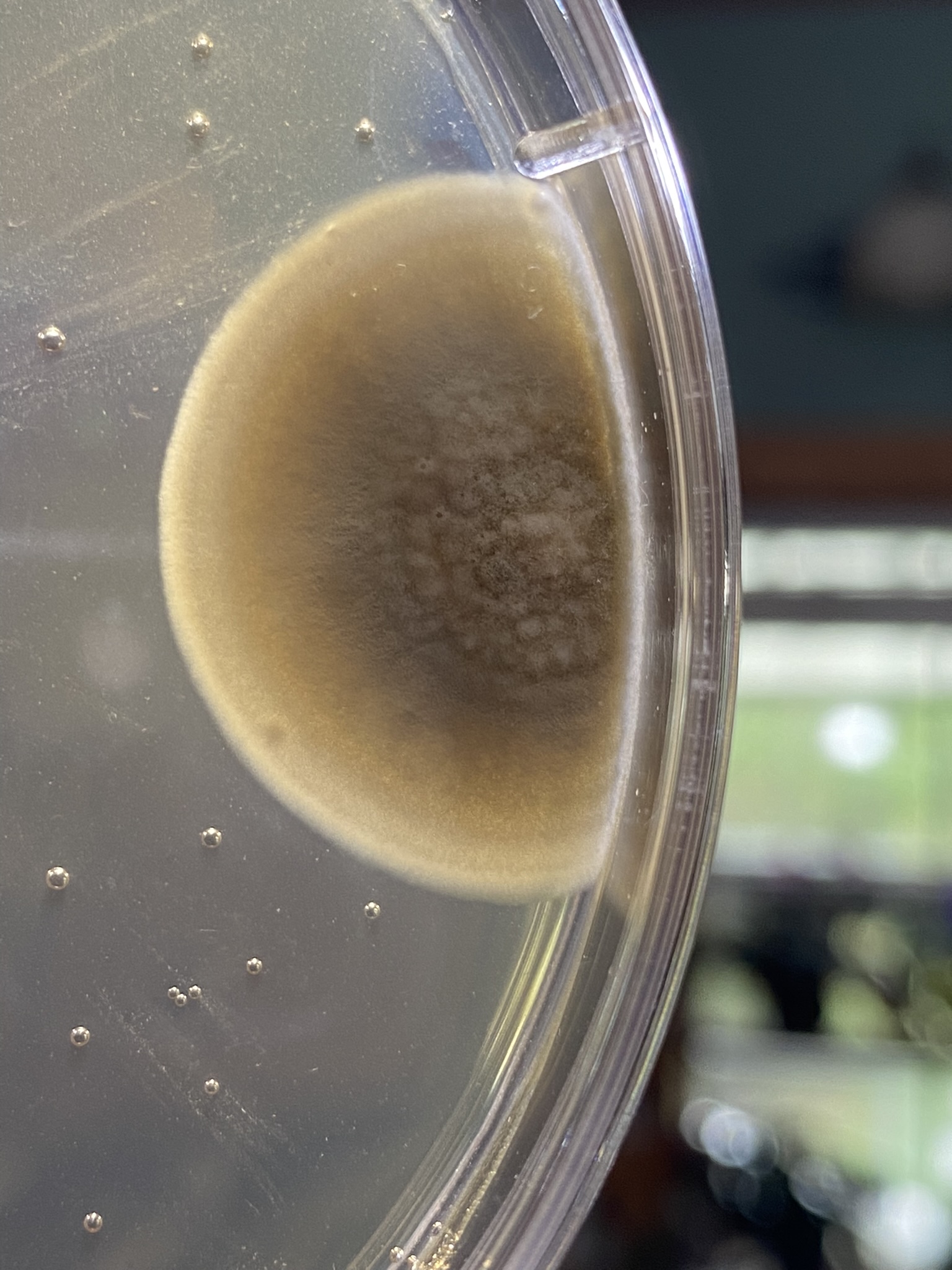
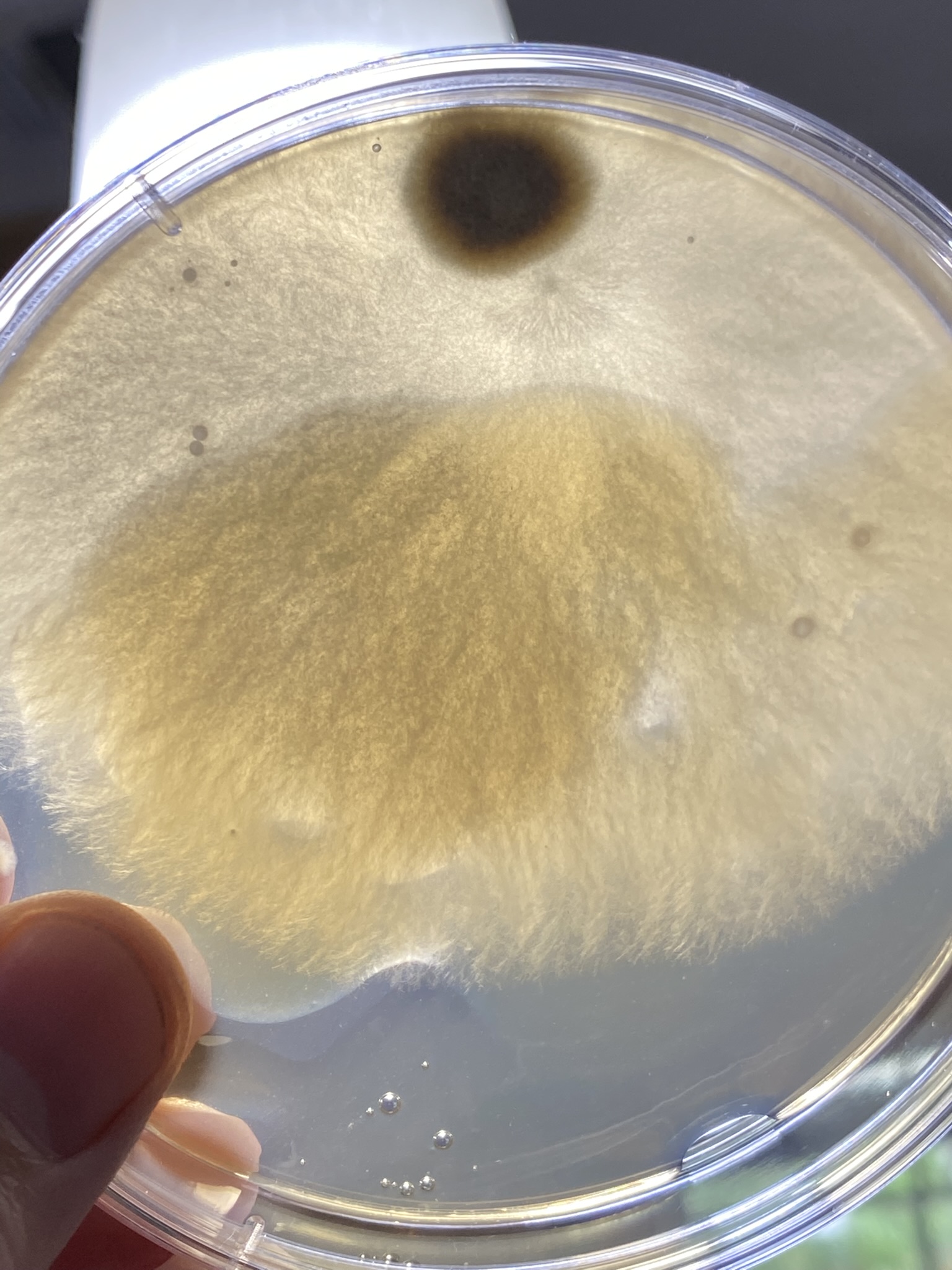
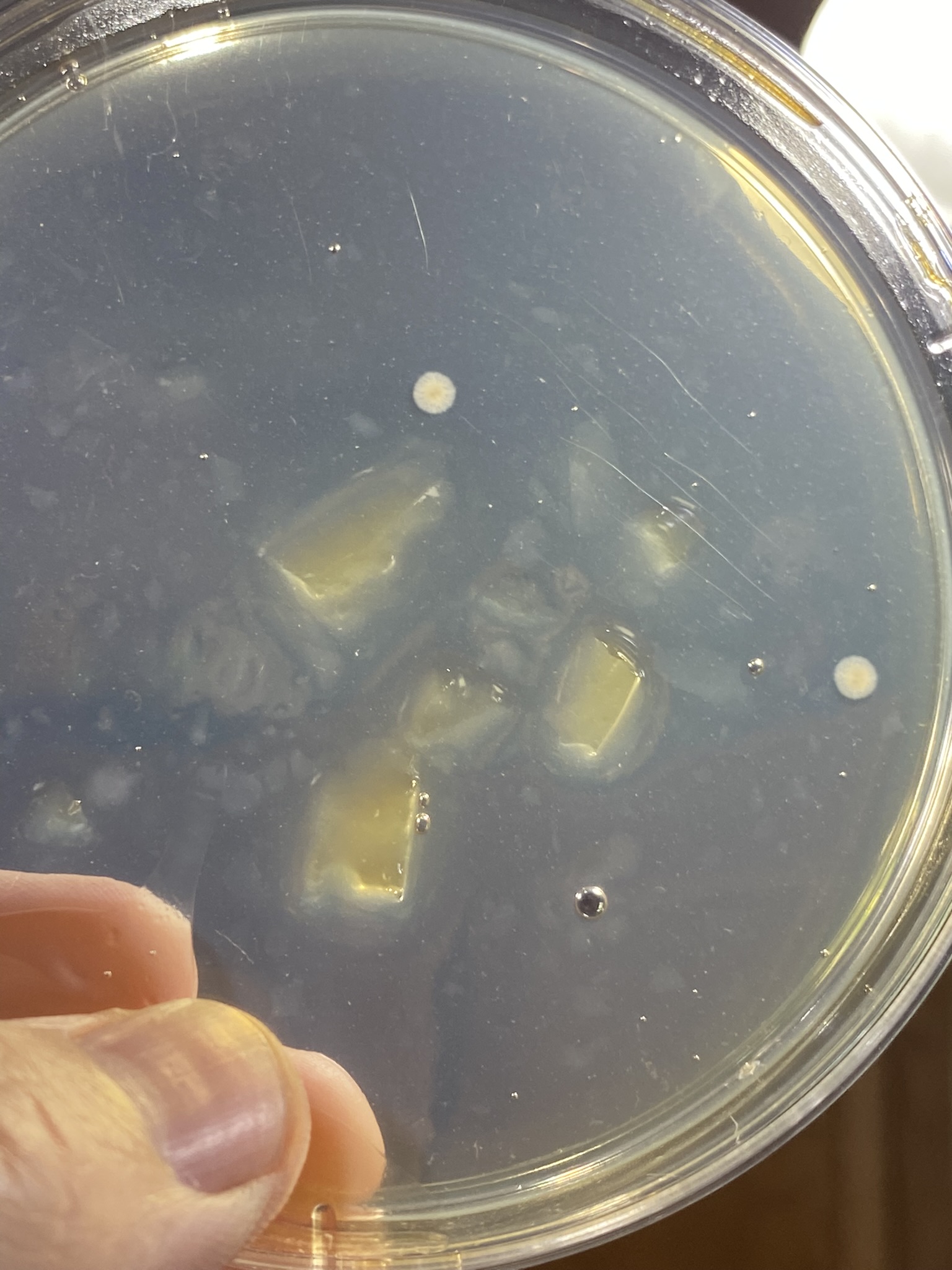
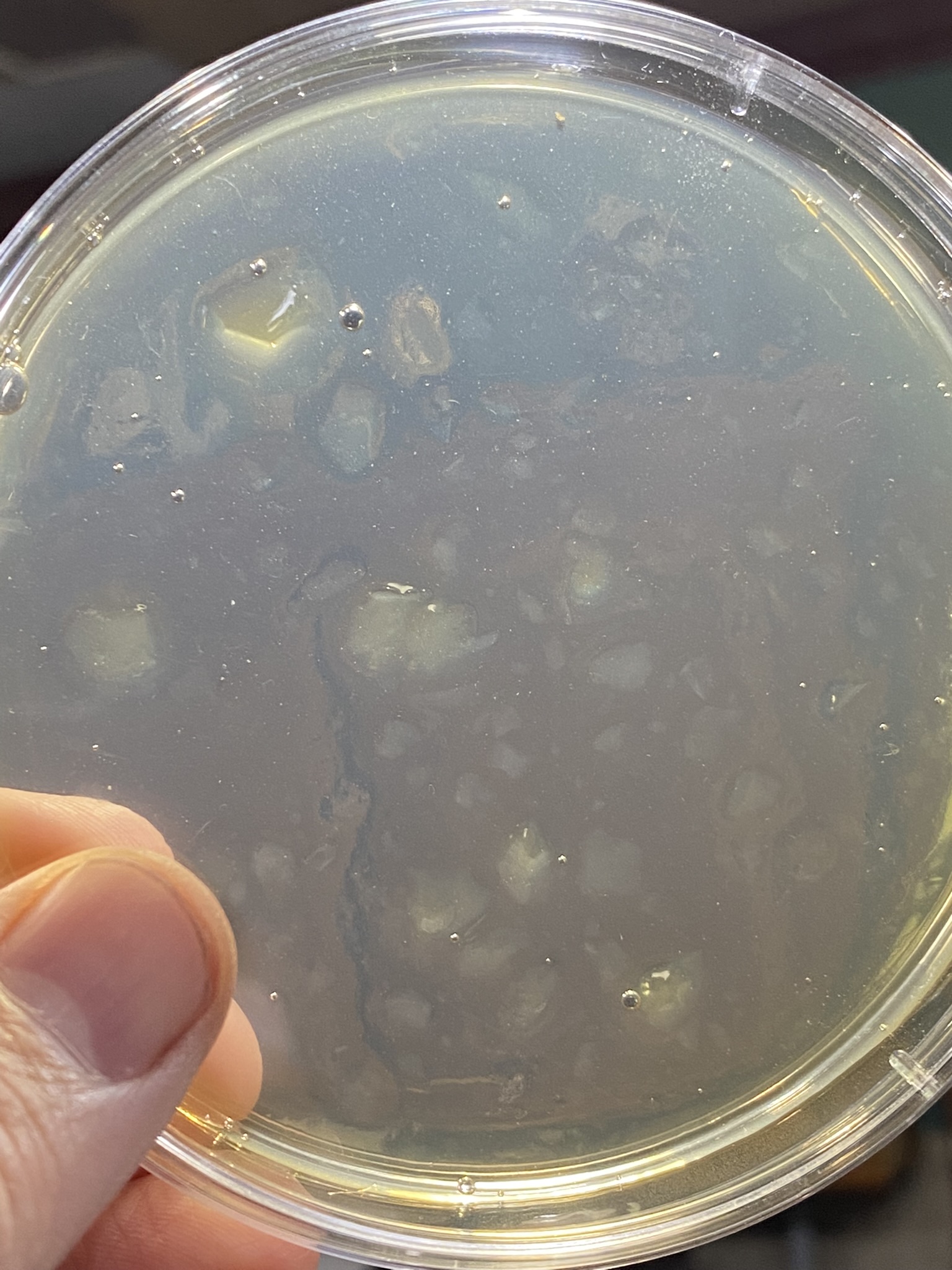
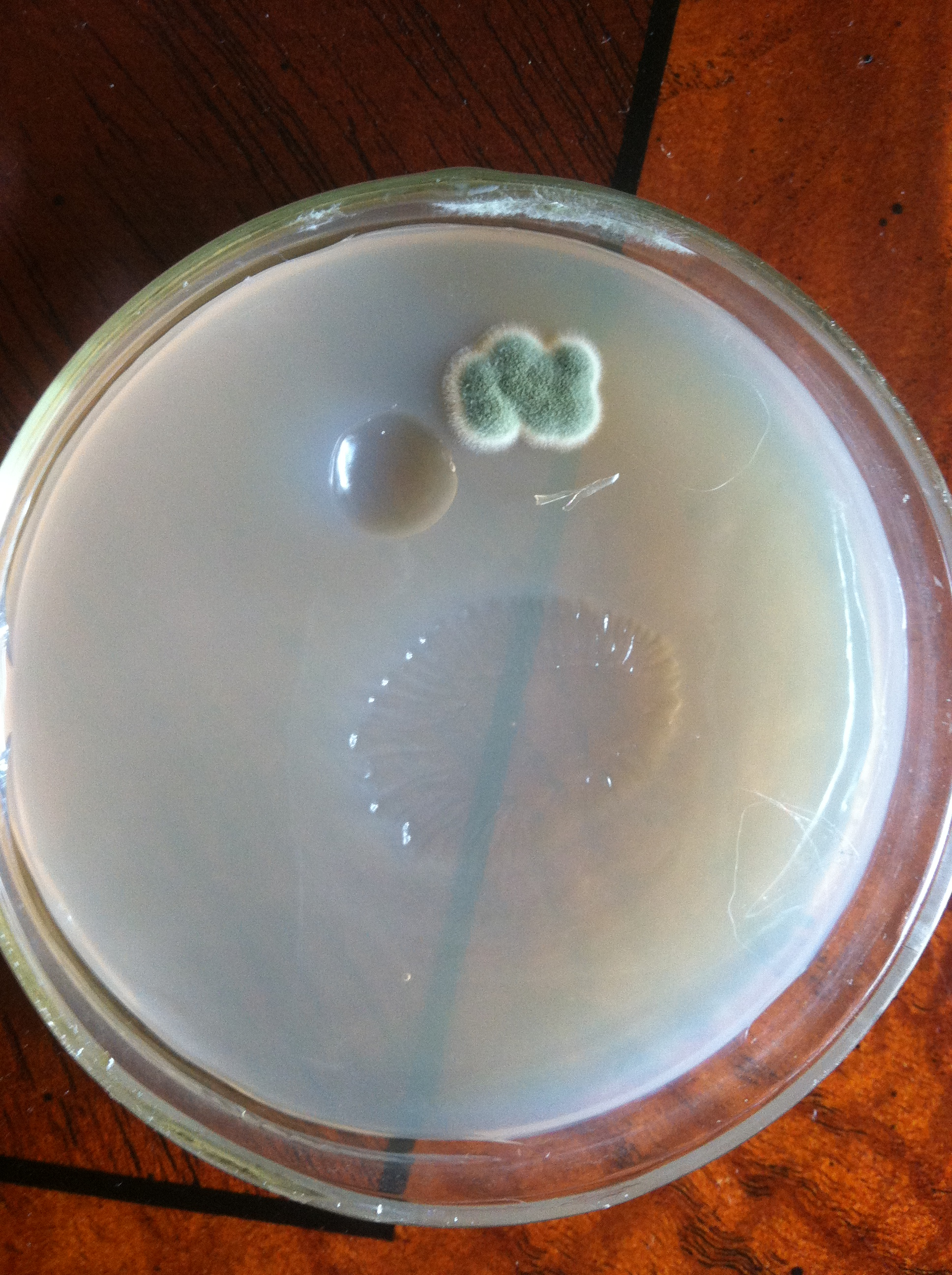
But first, maybe try and get a better sense of where you went awry on your sterile technique. Look at the contaminated plates, which I realize is difficult if they're in the trash. If the contaminants were all on the surface, then it's likely an issue with pouring. If you have buggies growing embedded in the agar, then your media and/or plates didn't start out sterile.
Personally, I don't take any stringent measures to work in a lab-clean environment. Just some basic common sense. Air has buggies. Fact of life. Absent working in a laminar flow lab hood, you're gonna have to live with that. And, guess what? Most folks in a lab pour their plates out on a bench top, not in a sterile environment. I certainly did.
How's that possible? Work smart. Find yourself a fairly clean area with a non-porous work surface, free of drafts. Avoid areas that are likely to be full of contaminants floating about and don't work in an area where you have a draft from a window or AC/heater air vent. If that's unavoidable, then turn off your AC or heater while you set up your plates. I work at my home office desk and time my pouring when the AC has cycled off.
Start with everything sterile. Make up your agar in a pourable, autoclavable (pressure cooker/canner) container. A glass bottle with a narrow neck and screw cap closure is going to work better for you than a canning jar. It's just hard to pour from a canning jar without some running down the side, which is going to greatly increase your chances of contaminating things. Another point in favor of that style of container is that you can reclose the bottle and still have sterile, usable agar for another day if you were careful. There's a very low chance of pulling that off with a canning jar. Just remember to keep that closure loose when you sterize it and to let your pressure cooker return to atmospheric pressure slowly, so you don't have explosive boiling of your agar.
Personally, I make the agar up ahead of time and let it sit around solidified until ready for use. I suggest you do the same. If something grows in the bottle, then you know you screwed up. If it stays clean, you're good to go.
Clean, sanitize, and sterilize your work area as best as you can. Do this before re-melting your agar. As I mentioned, I just use my office desk. Clear out some elbow room and wipe everything down with ethanol or a lysol solution. I'll often use a double wammy of starsan followed by high proof ethanol like ever clear. In the lab, we'd sometimes light it, but that's not wise at home. Probably overkill to sanitize and then finish off with ethanol, but I'd definitely err on the side of something better than just a brewing sanitizer alone.
Make sure the plates are actually sterile. Disposable plates are not autoclavable, as you now know. Buy them pre-sterilized if you can and store the stack with the plates in an inverted position. Dust has contaminants and dust settles down. Take out the plates you intend to use and reseal the sleeve. No dilly dallying and work in your cleaned work area.
I'd go as far as suggesting you hold your breath, as your own breath is likely the biggest source of contaminants if you're doing it right. Breath off to the side when you have to. That's a fairly important tip that applies to all the steps where you are working in the open. As covid has made most folks aware, even a normal breath carries aerosols with contaminants.
Wear disposable gloves. Probably wipe them down with starsan and/or ethanol, since gloves out of a box aren't sterile.
If in doubt about the sterility of your plates, give the plates a rinse with a bit of the everclear and shake off the excess. Put the lid down on your work area, face up, and prop the bottom of the plate on it, inverted, to dry. No drafts, no sudden movements, and no breathing when you have anything exposed. That desk chair you're probably sitting on is full of contaminants and you make a cloud with every movement. You can breath again (off to the side) when the plate is inverted, but try to not breath directly at anything you're working on. When they're dry, slide the bottom onto the lid, leaving the plates inverted. Tada. You now have (mostly) sterile plates. If you have pre-sterilized plates, skip this and just set your plates out on your work area, inverted.
When ready to plate, just loosen the cap on your bottle of agar (in a clean area since it will likely suck in air) and pop it into the microwave to melt. Heat until it's fully melted. Bring it to just a boil. Make sure that cap is loose. Do keep an eye on it or you'll have a helluva mess in your microwave.
Take your molten, near boiling, agar to your work area, where you have your plates ready to go. The hotter the agar is when your pour the plates, the more it will cover your lapses in technique. That said, there's no need to sprint or rush from the microwave to your work area.
Wearing your sterilized gloves, take the bottom of a plate and set it face up adjacent to the lid and pour your plate. Put the lid on as soon as possible, while everything is still hot. Repeat for each plate, but cover one before you go to the next. You'll get condensation. We'll deal with that later, but get the lid on as soon as you can. Leave the lid on until the agar sets.
Now that you've let the agar plates set, flip them over so they're sitting on their lids. Lift the bottom and prop it on your cleaned work surface and the inverted lid and let them finish cooling. This will also let some of that condensation on the lid dry out. Again, no drafts; no sudden movements; no breathing in the direction of the plates. Gently back away.
When everything is all cooled, set the inverted, propped up plates back in their lids. Your plates should stay inverted through this whole process. You can now relax.
Stack up your plates and leave them inverted. Put them off to the side for a couple days to dry out a bit and see if you managed to keep them sterile.
Tada. You have plates. Pat yourself on the back and have a beer.
I'll describe streaking plates and how to make your own loop for streaking plates with what you have lying around the house in another post.























![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)



























































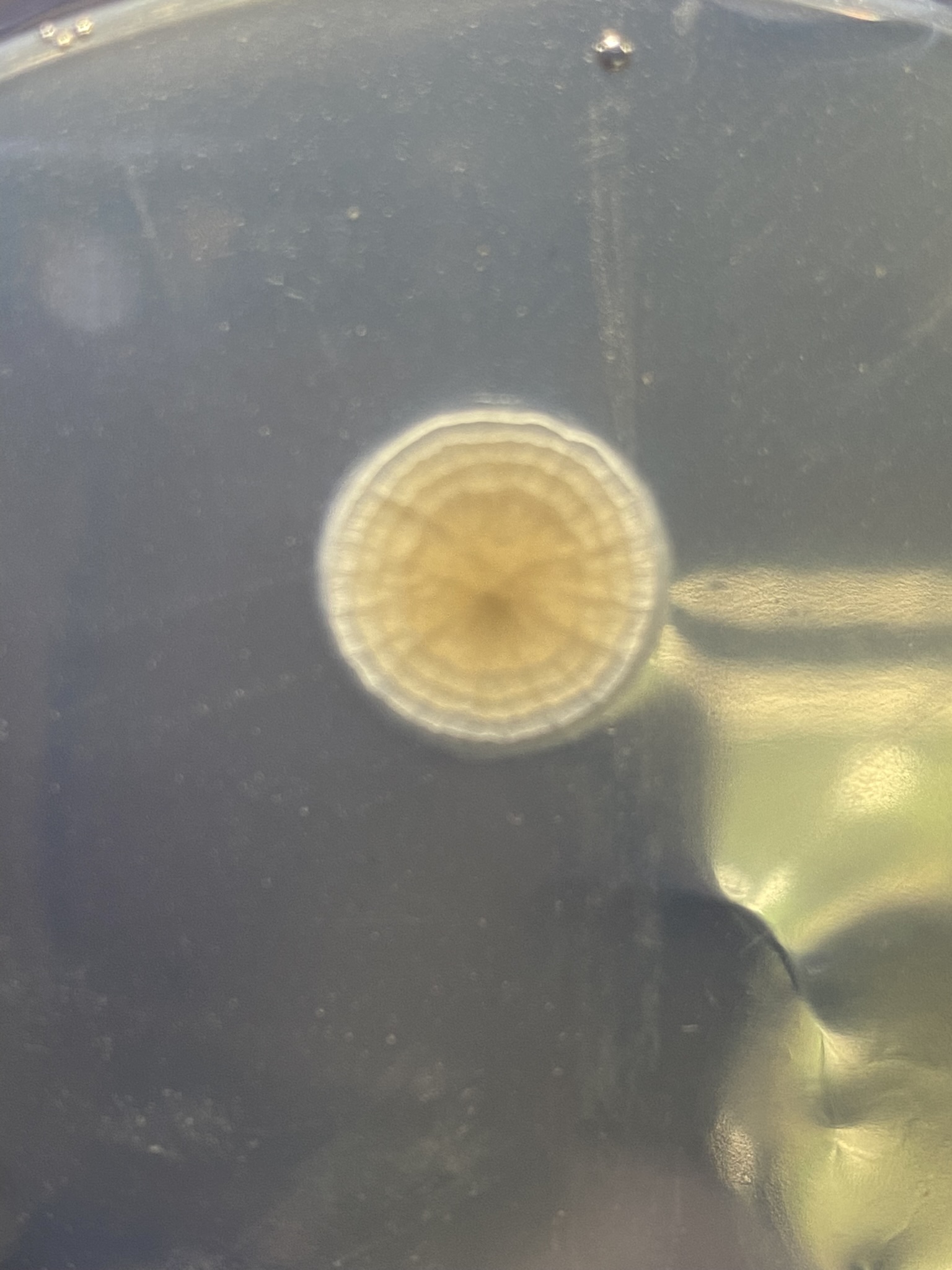


 Thank you for taking the time to give me such a detailed guide. I have read through your reply three times already to try to get things fixed in my mind.
Thank you for taking the time to give me such a detailed guide. I have read through your reply three times already to try to get things fixed in my mind.