@JONNYROTTEN and everyone else, i ask you to look up the action in which oxiclean does its thing before jumping to conclusions of what is or is not "safe."
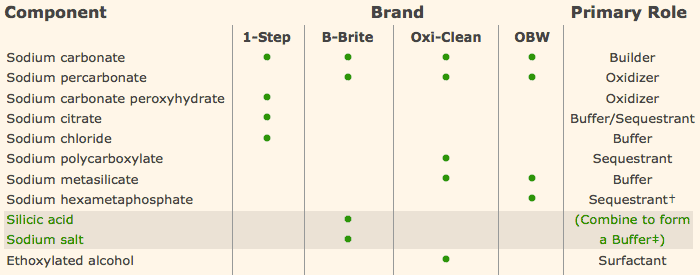
Sodium Percarbonate is the main ingredient in oxiclean. When mixed with water, it disassociates into hydrogen peroxide and sodium carbonate. Hydrogen peroxide is an oxidizer which is used as a disinfectant. It generally requires a contact time of 10min to disinfectant.
Now that we can consider sodium percabonate a disinfectant, we need to go back and evaluate the difference between a disinfectant and a sanitizer. Sanitizers kill 99.999% quickly, but disinfectants kill 100% slowly.
Disinfectant like oxi needs to be rinsed. So does bleach, so what, big deal, who cares. Unless you are using septic wate to rinse, you have nothing to be concerned about. Dump and Fill a plastic water bottle with your tap water and cap it. Leave it for a month. Anything grow? No, your fine to proceed.
But, but, what if i accidentally introduced germs when i used my garden hose from my yard to fill/rinse my container? Then it doesn't matter how aseptic your cleaning regime may be, you just contaminated after cleaning. Star-san or not you dun screwed the pooch in this.
Remember though, you don't need to be "sterile," you only need to create an environment where your chosen infection (yeast) can out compete the other microbes. Many of the peeps out here are over-complicating and over-thinking the process. Not saying that you are wrong, but pointing out that many of us shoot the horse after its already dead, just to be sure.
And for the record, I beat dead horses in my fermenting practices. The only infections I've had are from wild/open fermentations of wild vegetable greens where they are salt rinsed then left to ferment in their own brine. This was from incomplete submerging of the material and aerobic microbes began to reproduce. Never in a beer, wine or cider.









































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)















