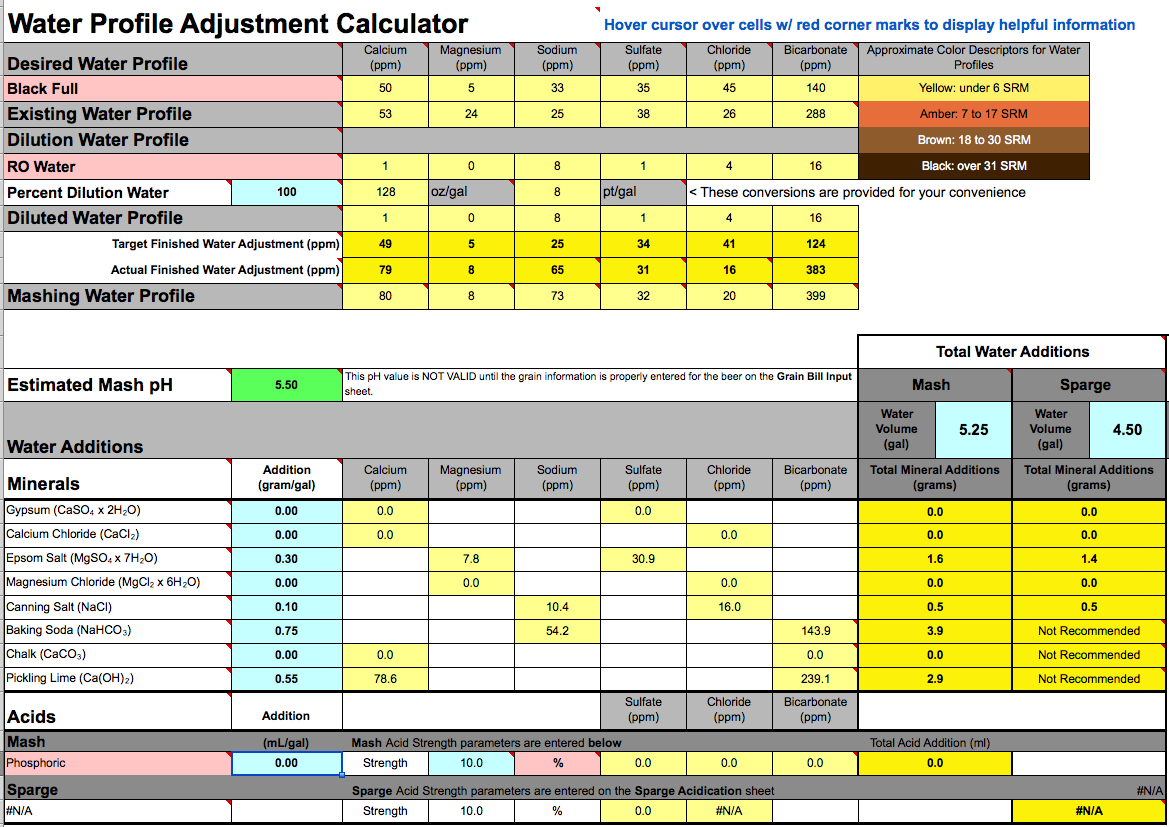
As I noted in #57 it is a peculiarity of Brun water that it represents acidity and alkalinity as bicarbonate which, while it is technically doable, confuses 99.99% of users as 99.999% of brewers and scientists do not use this representation. The other 0.001% know enough chemistry to figure out what he is doing. Most of the world uses mEq/L (a measure of the actual proton emission or absorption) and those who don't use a wide array of measures such as dH (German degrees), °F (French degrees) or, in the US, ppm as CaCO3. Bru'n water's representation is mEq/L expressed 'as bicarbonate'. To add to the confusion the 'as' is omitted which causes many to think that the number reported in the bicarbonate field actually represents the amount of bicarbonate ion in the water (which it does in many cases i.e. where the water is of pH < 8 and no acids or bases have been added to it - you got a negative bicarbonate indication because you added acid). Thus, if that field shows 30.5 the user is likely to conclude that there is 30.5 mg/L bicarbonate ion in the water even though there is, in fact, none (as if, for example, one added lime to distilled water). The number is, in fact, the alkalinity in mEq/L multiplied by 61, the equivalent weight of bicarbonate ion. Were this labeled 'as bicarbonate' the number of confused would probably go down to 99% as about one percent, being familiar with the 'as CaCO3' notation would know enough to divide the 'as bicarbonate' number by 61. Thus 30.5 mg/L 'bicarbonate' really means 30.5 mg/L 'as bicarbonate' which really means 30.5/61 = 0.5 mEq/L alkalinity (proton deficit). If you see a negative number, such as your -132, the frustration is going to be higher as you are asking probabky asking yourself 'how the hell can I have a negative amount of an ion?' But what it really means is -132/60 =2.2 mEq/L acidity (proton surfeit).
I recognize that there is a good chance that my explanation has confused you even more. IMO the bicarbonate value in Brun water is, for most people (the exception being the few users who understand the chemistry pretty well) worse than useless as it impairs, rather, then enhances understanding. I would, therefore, suggest (as you have done) that you ignore the bicarbonate number and focus on the target pH.








































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)