You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Slanting yeast
- Thread starter Saccharomyces
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
HollisBT
Well-Known Member
Yup. Slanting.
Sent from my iPhone using Home Brew
Sent from my iPhone using Home Brew
Slanting???
Sent from my iPhone using Home Brew
HollisBT, yetijunk and others, why do you think we all need to know that your phone is shiny, has a Apple logo on it and is manufactured in Chinese labour camps?
How is this related to brewing beer?
HollisBT, yetijunk and others, why do you think we all need to know that your phone is shiny, has a Apple logo on it and is manufactured in Chinese labour camps?
How is this related to brewing beer?
Wow! I thought I was the only one who finds these mobile "signatures" highly annoying. I wish they could be suppressed, which I bet, it's simply the app. generating that nonsense.
iDiots
SeanMcClellan
Active Member
Wow! I thought I was the only one who finds these mobile "signatures" highly annoying. I wish they could be suppressed, which I bet, it's simply the app. generating that nonsense.
iDiots
By default, the homebrewtalk app for iPhone has that signature turned on.
HollisBT
Well-Known Member
HollisBT, yetijunk and others, why do you think we all need to know that your phone is shiny, has a Apple logo on it and is manufactured in Chinese labour camps?
How is this related to brewing beer?
Because we need you to understand that we are better than you.
Clearly it's working.
Sent from my iPhone using Home Brew

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$719.00
$799.00
EdgeStar KC2000TWIN Full Size Dual Tap Kegerator & Draft Beer Dispenser - Black
Amazon.com

$479.00
$559.00
EdgeStar KC1000SS Craft Brew Kegerator for 1/6 Barrel and Cornelius Kegs
Amazon.com

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive

$44.99
$49.95
Craft A Brew - Mead Making Kit – Reusable Make Your Own Mead Kit – Yields 1 Gallon of Mead
Craft a Brew

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Gas MFL)
Guangshui Weilu You Trading Co., Ltd

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$20.94
$29.99
The Brew Your Own Big Book of Clone Recipes: Featuring 300 Homebrew Recipes from Your Favorite Breweries
Amazon.com

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$76.92 ($2,179.04 / Ounce)
Brewing accessories 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304 Brewing accessories(Gas Hose Barb)
chuhanhandianzishangwu

$159.99 ($26.66 / Count)
3M High Flow Series System BREW120-MS, 5616001, For Brewed Coffee and Hot Tea, Valve-in-Head Design
SpaceCityProviders

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司
SeanMcClellan
Active Member
I'm also curious if there is currently a phone on the market that isn't "manufactured in Chinese labor camps?" Not arguing, honestly curious.
How i agree! It (and its kind) has annoyd me since macebook started sending me invitations to join in the form of unknowns and oh, please quit asking/telling me to "Like you" on this or that site.
I am sure there must be a money trail somewhere but please- back off and drop dead.
just wanted to chime in and chant with you...
ohh-Kay...
My next post will try to get us back on topic!
I am sure there must be a money trail somewhere but please- back off and drop dead.
just wanted to chime in and chant with you...
ohh-Kay...
My next post will try to get us back on topic!
OK
I have begun to slant.
& I made a few boo-boos.
(probably nothing serious, but definately some things I want to get your input on!)
First, I made the culture solution in an opaque pot... long story short...
I ended up with protein junk in my slants!
It was all in a flurry of actions I am not really sure if I was expecting trouble or not, but IIRC I think I might have tried to filter thru a coffee filter... dont know if I saw some thing or if I even filtered... I can't even remember when I noticed the problem... after sterilization or after filling or several days later.... but the bottom line is
I got lots of marbled slants...
Q1
Exactly... what do I need to do next time to make sure this doesn't happen again?
Q2
Can you forsee any problem as a result of using the marbled slants?
(I considered starting over,
but thought perhaps it was only a cosmetic issue and that success could still be at hand.
Do you think I made the right call?)
I stored the slants quite a long time (weeks) at room temp... there was no growth;
they were sterile.
Finally - i got around to innoculating them.
2 days at room temp and ea slant had the growth I was expecting.
Q3
How long should I let them grow at RT ?
I am thinking that letting them grow a bit will give any bad guys present
a chance to show themselves
(off colors etc.)
Q4
What is the down side of NOT refridgerating them?
Q5
What % coverage of the surface do you aim for before cool storage?
Thanks guys...
SENT TO YOU by an amazing new bit of old technology the knowledge of which I have no deisre force upon you.
I have begun to slant.
& I made a few boo-boos.
(probably nothing serious, but definately some things I want to get your input on!)
First, I made the culture solution in an opaque pot... long story short...
I ended up with protein junk in my slants!
It was all in a flurry of actions I am not really sure if I was expecting trouble or not, but IIRC I think I might have tried to filter thru a coffee filter... dont know if I saw some thing or if I even filtered... I can't even remember when I noticed the problem... after sterilization or after filling or several days later.... but the bottom line is
I got lots of marbled slants...
Q1
Exactly... what do I need to do next time to make sure this doesn't happen again?
Q2
Can you forsee any problem as a result of using the marbled slants?
(I considered starting over,
but thought perhaps it was only a cosmetic issue and that success could still be at hand.
Do you think I made the right call?)
I stored the slants quite a long time (weeks) at room temp... there was no growth;
they were sterile.
Finally - i got around to innoculating them.
2 days at room temp and ea slant had the growth I was expecting.
Q3
How long should I let them grow at RT ?
I am thinking that letting them grow a bit will give any bad guys present
a chance to show themselves
(off colors etc.)
Q4
What is the down side of NOT refridgerating them?
Q5
What % coverage of the surface do you aim for before cool storage?
Thanks guys...
SENT TO YOU by an amazing new bit of old technology the knowledge of which I have no deisre force upon you.
the growing of yeast on agar, the surface of which forms an angle significantly unequal to 90 degrees with the sides of the container, by virtue of the agar solution solidifying in a test tube oriented in less than a totally upright position.
HollisBT
Well-Known Member
In an effort to save time when re-starting yeast, does anyone see a problem with sort of doubling up on how much yeast you start? For example, for a 10 gallon high gravity batch, could I grow enough cells using the following start up steps:
start three 10ml initial steps (24 hours)
add into 300ml of wort on stir plate (24 hours)
pitch to 3l starter on stir plate (36-48 hours)
Or possibly even breaking it down into three separate 100ml steps to pitch to the 3l step?
Any thoughts or input?
start three 10ml initial steps (24 hours)
add into 300ml of wort on stir plate (24 hours)
pitch to 3l starter on stir plate (36-48 hours)
Or possibly even breaking it down into three separate 100ml steps to pitch to the 3l step?
Any thoughts or input?
In an effort to save time when re-starting yeast, does anyone see a problem with sort of doubling up on how much yeast you start? For example, for a 10 gallon high gravity batch, could I grow enough cells using the following start up steps:
start three 10ml initial steps (24 hours)
add into 300ml of wort on stir plate (24 hours)
pitch to 3l starter on stir plate (36-48 hours)
Or possibly even breaking it down into three separate 100ml steps to pitch to the 3l step?
Any thoughts or input?
That scheme should work. All your steps are within the 10x increase recommended by Chris White and others. The only issue I can see, making 3 initial 10ml cultures triples your chance for contamination at the most critical step.
That said, I have been looking into and experimenting with inoculation rates for step increases. With all due respect for the 10x increase between steps there are other camps out there that would recommend a smaller fold increase, i.e. a higher inoculation rate. Somewhere I came across the 4 - 7 rule. I think it is practiced in Europe. Anyway, I have tried this with great success. The 4 - 7 rule says, step increases should be no less than 4x and no more than 7x. As it turn out, by following this rule, my steps are in log phase within 12 - 14 hours at 70 F under good aeration. Certainly there will be strain differences but my Belgian strains are typically ready in 12 hours. This saves a lot of time for preparation (even with the fact you may need an extra step) and allows a smaller window for any contamination to increase.
So consider this scheme:
Start a 10ml initial step (12 -24 hrs) 2 big loop-fulls from a fresh slant will be in log phase in 12 hrs, older cultures may require more time. Even better, start a few days earlier (4 for my strains) by dilution streaking from your slant to a plate and pick 4 -6 single colonies (you can be sure these single colonies are contamination free and are in great health).
Add to 60ml for a total of 70 ml (12 hrs)
Add to 400ml for a total of 470ml (12 hrs)
Pitch to 3l for a total of 3470ml (12 hrs to log phase)
A word of caution on pitching cultures grown from slants: This is practically the same method yeast labs use to produce yeast. Lab produced yeast is the healthiest most viable yeast possible and therefore require a lower pitching rate. And because you are producing them in your own lab, they are the freshest yeast possible. White Labs and Wyeast both recommend pitching about half the rate of lab produced yeast compared to re-pitching from a previous batch. So on average you would want to pitch 500,000 cells/ml/degree Plato. So for a 1.060 10 gallon batch you should pitch 283 billion cells. I increase that to 600,000 cells/ml/degree Plato for my higher gravity Belgian ales (~1.075). If you were doing a big lager you may want to use 750,000 cells/ml/degree Plato.
Concerning yeast yield when growing up from slants: Your yield may be much higher than what yeast calculators would predict. In the literature and my experience you can produce as much as 300 million cells per ml in a well aerated culture at 75 degrees F. 200 to 250 million may be good estimate if you can't count cells but estimate less if you are in doubt about your aeration. If your stir plate can make a vortex that touches the stir bar, estimate 200 to 250 million cells per ml. I just scored an orbital shaker and my last count was 292 million cells per ml!
So at 250 million cells per ml, your 3470ml culture would contain 867 Billion cells!
I'm saying all this because I learned the hard way. I kept building bigger and bigger cultures thinking I was under-pitching but my Belgians just kept getting cleaner and cleaner, lacking the esters I was looking for. After I gained the ability to count cells, I realized I was over-pitching by >2x.
OK
I have begun to slant.
& I made a few boo-boos.
(probably nothing serious, but definately some things I want to get your input on!)
First, I made the culture solution in an opaque pot... long story short...
I ended up with protein junk in my slants!
It was all in a flurry of actions I am not really sure if I was expecting trouble or not, but IIRC I think I might have tried to filter thru a coffee filter... dont know if I saw some thing or if I even filtered... I can't even remember when I noticed the problem... after sterilization or after filling or several days later.... but the bottom line is
I got lots of marbled slants...
Q1
Exactly... what do I need to do next time to make sure this doesn't happen again?
Q2
Can you forsee any problem as a result of using the marbled slants?
(I considered starting over,
but thought perhaps it was only a cosmetic issue and that success could still be at hand.
Do you think I made the right call?)
I stored the slants quite a long time (weeks) at room temp... there was no growth;
they were sterile.
Finally - i got around to innoculating them.
2 days at room temp and ea slant had the growth I was expecting.
Q3
How long should I let them grow at RT ?
I am thinking that letting them grow a bit will give any bad guys present
a chance to show themselves
(off colors etc.)
Q4
What is the down side of NOT refridgerating them?
Q5
What % coverage of the surface do you aim for before cool storage?
Thanks guys...
SENT TO YOU by an amazing new bit of old technology the knowledge of which I have no deisre force upon you.
Q2 first: I think what you are describing is break material and it should not cause any problem with your slants or growth (as you already know) or storage.
Q1: Although the break material is not a problem, like you, I don't like it in my slants and worse in my plates. You can remove most of the break material by letting your wort cool before you add the agar and decanting the wort off the break. However there may be more break form when you sterilize but it should be at a minimum and you will have fairly clear slants. Another option is to consider canning your wort. I like this option for a number of reasons including clear media. About once a year I set aside a day to can 10 gallons of wort. I actually brew up a 10 gallon batch of 1.04 wort and remove the hot and cold break, after the brewing process, the cooled wort is drained into half gallon, quart, and some pint sized canning jars and pressure cooked for 20 minutes. After the the jars cool, more break material will have formed and stetted to the bottom of the jars. Later, when preparing media, the wort is decanted off of the remaining break material and very clear slants and plates can be poured. I don't worry too much about the break for the culture media as I understand that some break is good for the yeast.
Q3: I like to just give mine a couple of days. As soon as growth is apparent they go in the fridge as cold as possible. Keep in mind that yeast on slants are still metabolizing (even at 32 F) and don't need a lot of competition.
Q4: See Q3. Make an extra and leave it out. You may be surprised at the response. The yeast will think they are in a batch of wort. If you tighten the cap, it might blow up!
Q5: I like to see about 30 - 40% coverage. I'm not sure how you streak onto your slant but for me I don't want solid coverage so I make one line down the center of the slanted agar surface then make a repeating S from the top the bottom of the surface so that as the yeast grow it forms a series of connected dollar signs ($).
Most of my slants are now a year old, so it's time to re-slant them. Which leads me to two questions.
1) Should I plate out from the existing slants first, or simply slant to fresh media?
2) If my existing slants are Gen X, do they become Gen Y when re-slanted. I assume not, and figure the Generation only increments when they are used and grown in a starter & then wort.
1) Should I plate out from the existing slants first, or simply slant to fresh media?
2) If my existing slants are Gen X, do they become Gen Y when re-slanted. I assume not, and figure the Generation only increments when they are used and grown in a starter & then wort.
tally350z
Well-Known Member
No simply go from slant to slant. The purpose of the plate is to make sure you have a pure yeast culture that is not contaminated, and get a single celled culture. Also no on your second question, for me I only think of different generations when the yeast go through the streesses of a fermentation.
Edit for Blue Frogs question. I use parafilm on all my slant and plates. I am sure its not nessecary on the slants when they are sealed, but it only takes a little tiny piece for some extra peace of mind.
Edit for Blue Frogs question. I use parafilm on all my slant and plates. I am sure its not nessecary on the slants when they are sealed, but it only takes a little tiny piece for some extra peace of mind.
Edit for Blue Frogs question. I use parafilm on all my slant and plates. I am sure its not nessecary on the slants when they are sealed said:What size (width) is your parafilm?
What else can I seal with the 5cm size?
50 or 100ml beakers?
tkx
bf
Aunt_Ester
Well-Known Member
I use Saran Wrap as a sub for para film with good results. Remember to stretch it as you go around the plates circumference to lock it in place. A little goes a long way.
Sent from my iPhone using Home Brew
Sent from my iPhone using Home Brew
brewski09
Well-Known Member
No simply go from slant to slant. The purpose of the plate is to make sure you have a pure yeast culture that is not contaminated, and get a single celled culture.
Then why even reslant? I am under the impression that tesla ting is done to ensure viable yeast, in which case you would want to grow it up, test attenuation (preferably on a beer because why not), plate from the starter and slant from there. My concern would be losing attenuation from just reslanting 1 year old yeast.
Disclaimer, I'm not an authority on this. Just doing some thinking on the matter and want a good answer. If anyone has a technical link, I'd love to have it so I can read up on yeast viability when direct slanting.
Sent from my iPhone using Home Brew
Thanks trentm!
I think I get it now. I like your Be-Pre-Prepared method.
Do you tape/parafilm your (autoclavable capped) slant tubes?
(I'm only planning on doing that for plates...)
Thanks again,
BF
It would surely provide extra security. Air is your enemy when storing on slants or plates. And, I once lost a culture because I did not tighten the cap. That said, no I don't parafilm or tape my slants. For short term storage on plates, I use good quality electrical tape.
I am plannining on using color coded elect. tape on the plates... but I do fear sticky gooey residue... what is your concern over good quality? I was planning on the cheap Chinese stuff, unless you know of issues....
Most of my slants are now a year old, so it's time to re-slant them. Which leads me to two questions.
1) Should I plate out from the existing slants first, or simply slant to fresh media?
2) If my existing slants are Gen X, do they become Gen Y when re-slanted. I assume not, and figure the Generation only increments when they are used and grown in a starter & then wort.
No simply go from slant to slant. The purpose of the plate is to make sure you have a pure yeast culture that is not contaminated, and get a single celled culture. Also no on your second question, for me I only think of different generations when the yeast go through the streesses of a fermentation.
Edit for Blue Frogs question. I use parafilm on all my slant and plates. I am sure its not nessecary on the slants when they are sealed, but it only takes a little tiny piece for some extra peace of mind.
Then why even reslant? I am under the impression that tesla ting is done to ensure viable yeast, in which case you would want to grow it up, test attenuation (preferably on a beer because why not), plate from the starter and slant from there. My concern would be losing attenuation from just reslanting 1 year old yeast.
Disclaimer, I'm not an authority on this. Just doing some thinking on the matter and want a good answer. If anyone has a technical link, I'd love to have it so I can read up on yeast viability when direct slanting.
Sent from my iPhone using Home Brew
Three conditions you may want to consider when time to re-store your yeast; 1)Yeast in storage can mutate over time., 2) Possible contamination, and 3) poor yeast health. All these can be managed to some extent by dilution streaking to form single colonies before re-storing onto a slant.
One of the most common mutations is called "Petite mutants" or "respiratory mutants". As the name implies this type of mutant will form small colonies on a plate. If you have a large percentage of small colonies, your isolate may have problems. Be aware that large colonies often form as a result of growth from 2 or more cells. Typically in a healthy dilution streak plate you will see a few large colonies and numerous smaller (average size) colonies. It's the ones that are smaller than the average colonies that could be of concern.
Your slant could be contaminated and you can't see it. By streaking from your slant for single colonies, many possible contaminates would become visible. If contamination is spotted, all is not lost. You may be able to select a clean colony for re-storage. In this case, it may take least one or more rounds of re-isolation to be sure you have purified your isolate and a fermentation test may be advised.
For sure your year-old yeast are tired, hungry and in poor health. Think of a hibernating bear. Upon awakening, the first thing she wants to do is build back her reserves. By spreading out your yeast on a agar plate, they have plenty of room to get all the nutrients and sugars they need to from healthy new cells that are ready to go back to sleep and get ready for their next job.
I am plannining on using color coded elect. tape on the plates... but I do fear sticky gooey residue... what is your concern over good quality? I was planning on the cheap Chinese stuff, unless you know of issues....
For me it's easier to use (more flexible) and sticks better. Even with the good stuff, it often comes loose at the end in cold conditions. Like earlier advised with saran wrap or parafilm, it seems to work and seal better if stretched as applied. I don't remember gooey residue with the cheaper stuff but IMO the good stuff is worth the extra $ and does a lot of plates.
brewski09
Well-Known Member
Three conditions you may want to consider when time to re-store your yeast; 1)Yeast in storage can mutate over time., 2) Possible contamination, and 3) poor yeast health. All these can be managed to some extent by dilution streaking to form single colonies before re-storing onto a slant.
One of the most common mutations is called "Petite mutants" or "respiratory mutants". As the name implies this type of mutant will form small colonies on a plate. If you have a large percentage of small colonies, your isolate may have problems. Be aware that large colonies often form as a result of growth from 2 or more cells. Typically in a healthy dilution streak plate you will see a few large colonies and numerous smaller (average size) colonies. It's the ones that are smaller than the average colonies that could be of concern.
Your slant could be contaminated and you can't see it. By streaking from your slant for single colonies, many possible contaminates would become visible. If contamination is spotted, all is not lost. You may be able to select a clean colony for re-storage. In this case, it may take least one or more rounds of re-isolation to be sure you have purified your isolate and a fermentation test may be advised.
For sure your year-old yeast are tired, hungry and in poor health. Think of a hibernating bear. Upon awakening, the first thing she wants to do is build back her reserves. By spreading out your yeast on a agar plate, they have plenty of room to get all the nutrients and sugars they need to from healthy new cells that are ready to go back to sleep and get ready for their next job.
That's some great information. Thanks. To re-ask a question just up thread do you consider it a new generation of yeast if you take it from slant to plate?
Sent from my iPhone using Home Brew
That's some great information. Thanks. To re-ask a question just up thread do you consider it a new generation of yeast if you take it from slant to plate?
Sent from my iPhone using Home Brew
Well, I didn't answer earlier because I really don't know. But I'll give you my thoughts. As Tally350z says, "I only think of different generations when the yeast go through the streesses of a fermentation." I would call that Fermentation Generations. If you are re-pitching yeast, it is very important to keep up with how many generations the yeast have been used. That would allow you to better manage re-pitched yeast. Likewise, if one is re-storing yeast it would be considered good management to keep track of how many time the yeast has been re-stored. So I guess you could call each re-storing a "generation". However, if you are also re-pitching yeast, I would keep those "generations" separate. I would call the first pitch to a fermentation the "first generation" regardless how many times that yeast has been re-stored.
ColoHox
Compulsive Hand Washer
As long as you are consistent with your batch/generation/pass recording, you can use any approach that you like. Either just record fermentations, or record every time that the culture is stored or grown, etc. Here is a handly flowchart I designed to demonstrate the different recording methods:


ColoHox
Compulsive Hand Washer
???
Maybe I'm following less visually. Can you define your terms?
stock
passage
batch
generation
What is the dif. btw. stock A & stock B
Is B a backup ?
Where does a reslant occur in this scheme?
That's fair. I am showing that the terms can be used in different ways. A stock would be storage (slant/frozen/washed), a "batch" of beer (round of full fermentation), then passage and generation are nearly interchangeable.
The labels (stock A&B) are just ways to keep track of the stored cultures, it is good practice to make more than one stock. So in the right side of the scheme, one of the stocks originally generated from the first starter (stock B, out of many stored cultures, ie more than just A and B) is used to ferment a batch. From that batch, yeast is washed (and/or slanted, frozen, etc) and split into two new stocks (B1, etc), then a new stock is used to ferment a new batch.
In my yeast farming practice, I follow the left side. From the original source, I make 10-15 working stocks. Each stock then ferments 2-5 batches, depending on the beer. On my last stock, I generate 10-15 more working stocks, all of which are a new generation than the previous batch of stocks, which is taken into account when fermenting new batches.
Is that more clear?
HollisBT
Well-Known Member
Has anyone tried to, or been successful at, slanting lager yeast?
And if so, how do you adjust your propagation steps? Simply allow longer (two to three times as long?) intervals for growth?
Sent from my iPhone using Home Brew
And if so, how do you adjust your propagation steps? Simply allow longer (two to three times as long?) intervals for growth?
Sent from my iPhone using Home Brew
Unless slanting at near freezing temperatures, perhaps such an increase is not needed. Grown at the same temps, growth rates of different yeasts seem roughly the same to me.... (limited observation).
Just did this, thanks for all the help. Used telephone brand agar, available on ebay for cheap. 7.5g in 500ml of wort.
http://imgur.com/a/a3FTo
http://imgur.com/a/a3FTo
cyberbackpacker
Well-Known Member
Just did this, thanks for all the help. Used telephone brand agar, available on ebay for cheap. 7.5g in 500ml of wort.
http://imgur.com/a/a3FTo
Nice photo documentation!

Thanks lol, nothing that isnt already on this great thread, but why not add more pics.
I pushed it to our club site with a bit better write up, http://laramiebrew.club/2014/10/yeast-slanting/
I pushed it to our club site with a bit better write up, http://laramiebrew.club/2014/10/yeast-slanting/
Do you think I could reanimate some slanted yeast in a tube of 40ml wort? I usually use this as my second step, with the first being 10 - 15ml. Each step last for about 24 hours so I was thinking of leaving the 40ml tube for 48 hours maybe with a few extra drags from the slant. Would this work?
HollisBT
Well-Known Member
Hey guys, I recently slanted a culture of 1217, and had after inoculating my media and letting the culture grow, I got some pretty heavy condensation on the inside of my tubes. The yeast looks very bright white and healthy on the media, and in sure that the condensation is nothing to worry about, but any input here? I just sealed the tubes and put them in my fridge, should I take them back out and loosen the caps until it dries completely?
FredTheNuke
Well-Known Member
I would leave them be. Yeast get thirsty too!
Thanks lol, nothing that isnt already on this great thread, but why not add more pics.
I pushed it to our club site with a bit better write up, http://laramiebrew.club/2014/10/yeast-slanting/
I'm pretty sure I'm most impressed with the "balled up foil" improv. Not sure why I didn't think of that. Thanks.
deeringbrewing
Active Member
- Joined
- Dec 1, 2014
- Messages
- 31
- Reaction score
- 2
Hey all. What are your procedures for plate streaking? Are you using a pure yeast colony on the loup to streak or are you diluting a pure colony in sterile water first? I'm just getting into streaking to get a pure strain and rid bacteria. I tested using a pure colony on the loup yesterday, I have been diluting first but I was getting varied results.
Some of my buddy's budding.
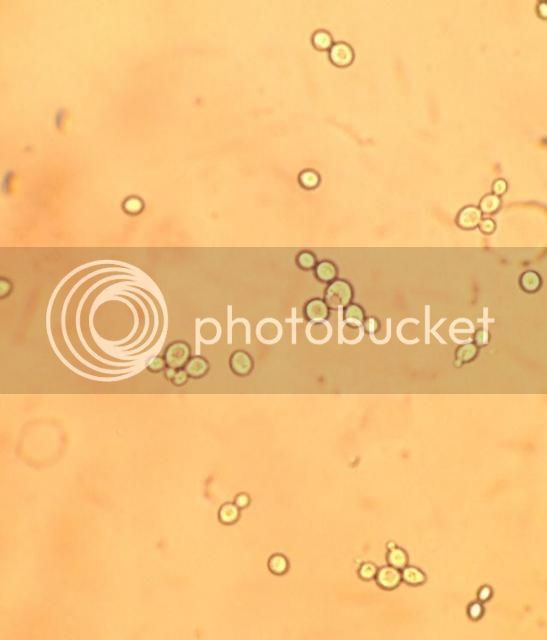
Some of my buddy's budding.

Similar threads
- Replies
- 8
- Views
- 3K
- Replies
- 3
- Views
- 750
- Replies
- 17
- Views
- 2K

























![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)
















