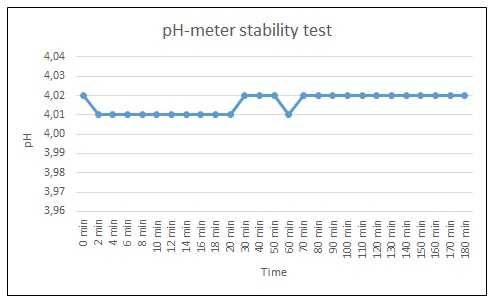
UPDATE 8/9/16: It turns out I received a bad batch of pH buffers from Atlas Scientific, leading the the calibration errors. The Hach meter was not to blame after all.
I'm planning on brewing tomorrow, so tonight I decided to try out my Hach Procket Pro+ for the first time to test the pH of my water after my additions. FWIW, I work in a lab and use a laboratory pH meter on a weekly basis.
First observation: as mentioned in an earlier post, the Pocket Pro+ box arrived covered in some sticky/slimy substance which I later determined to be soap. I purchased the meter through Hach's Amazon storefront, but as it was fulfilled by Amazon I can't say if this is Hach or Amazon's fault. Strange and a little unsettling, but everything inside the box was unaffected.
The included instructions don't contain any information about probe storage, but they direct you to the online manual, which is in the form of a PDF. This manual does not have any storage information either, so it's not really clear if the probe is intended to be stored wet, as with most meters, or if it matters at all.
I set out to perform a three point calibration using brand new calibration solutions, at room temperature (72F). The sensor and sample cup were rinsed with distilled water and wiped dry with Kimwipes before calibration and in between each step. The first calibration attempt did not succeed. After calibration completed, "SENSOR?" was flashing on the screen. According to the manual, this has something to do with either a dirty sensor or a calibration slope plus/minus 10-15%. I had cleaned the sensor before starting, but decided to try again.
I attempted to perform two more calibrations after the first. The second calibration resulted in "ECAL" and the calibration icon flashing with a question mark next to it. This indicates a calibration slope greater than plus/minus 15%, according to the manual. The third calibration attempt produced the same results as the first, with "SENSOR?" flashing on screen.
Other observations: the paint for the fill line on the sample cup more resembles a dry-erase marker than anything permanent. Having used the meter one time it has smeared and started to come off. I suspect it doesn't stand up to the calibration solutions. The fill level isn't critical as long as the sensor is submerged, so this isn't a huge deal.
So, it looks like I received a dud.
I'm planning on brewing tomorrow, so tonight I decided to try out my Hach Procket Pro+ for the first time to test the pH of my water after my additions. FWIW, I work in a lab and use a laboratory pH meter on a weekly basis.
First observation: as mentioned in an earlier post, the Pocket Pro+ box arrived covered in some sticky/slimy substance which I later determined to be soap. I purchased the meter through Hach's Amazon storefront, but as it was fulfilled by Amazon I can't say if this is Hach or Amazon's fault. Strange and a little unsettling, but everything inside the box was unaffected.
The included instructions don't contain any information about probe storage, but they direct you to the online manual, which is in the form of a PDF. This manual does not have any storage information either, so it's not really clear if the probe is intended to be stored wet, as with most meters, or if it matters at all.
I set out to perform a three point calibration using brand new calibration solutions, at room temperature (72F). The sensor and sample cup were rinsed with distilled water and wiped dry with Kimwipes before calibration and in between each step. The first calibration attempt did not succeed. After calibration completed, "SENSOR?" was flashing on the screen. According to the manual, this has something to do with either a dirty sensor or a calibration slope plus/minus 10-15%. I had cleaned the sensor before starting, but decided to try again.
I attempted to perform two more calibrations after the first. The second calibration resulted in "ECAL" and the calibration icon flashing with a question mark next to it. This indicates a calibration slope greater than plus/minus 15%, according to the manual. The third calibration attempt produced the same results as the first, with "SENSOR?" flashing on screen.
Other observations: the paint for the fill line on the sample cup more resembles a dry-erase marker than anything permanent. Having used the meter one time it has smeared and started to come off. I suspect it doesn't stand up to the calibration solutions. The fill level isn't critical as long as the sensor is submerged, so this isn't a huge deal.
So, it looks like I received a dud.
























![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)