jgodfrey
Member
- Joined
- Aug 10, 2014
- Messages
- 13
- Reaction score
- 2
Hi All,
I have a Ward Labs report for my tap water and I'd like to dilute it with some R/O water to tone down some of the compounds. I also have a report for the R/O water (from the provider) that I'll assume is accurate for my purposes.
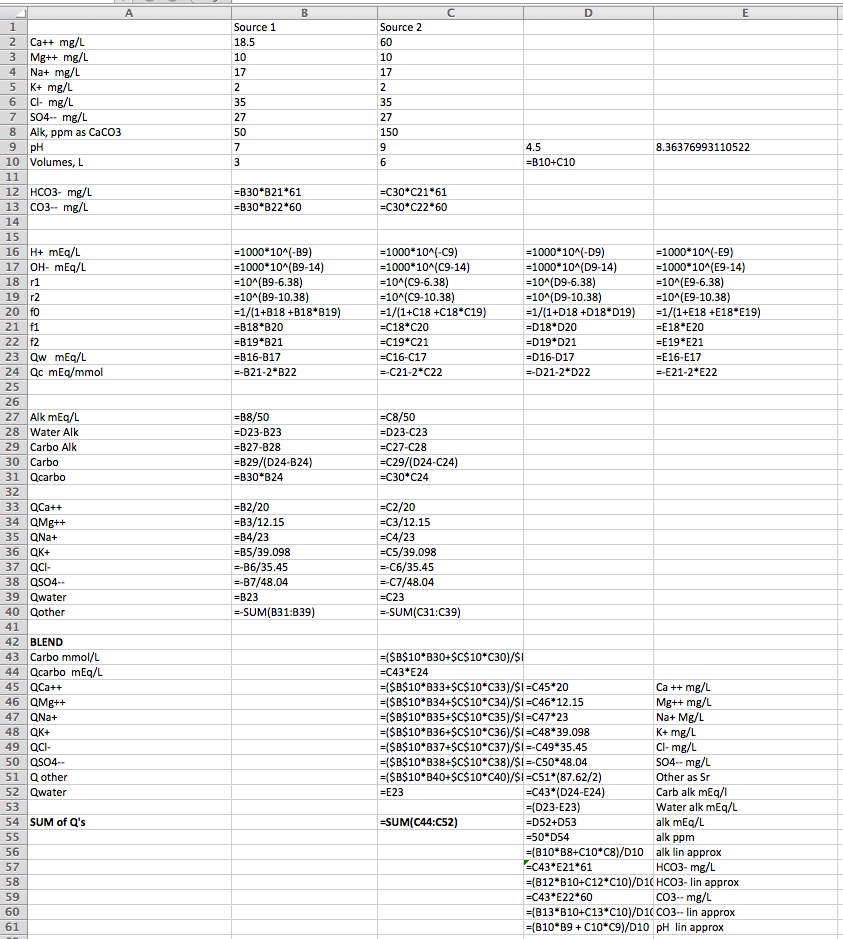
I assume that calculating the compounds resulting from a mix of the two waters is just a direct, linear relationship based on the percentages of the 2 waters in the final mix, right?
For example, if water A is 50 Na and water B is 30 Na than a 50/50 mix of the two waters will be 40 Na ( (50+30)/2 ), right?
From a mathematical perspective, that makes perfect sense to me, but I wanted to make sure there's not some other chemistry-based magic going on here.
Assuming the above is correct, does that hold true for final pH values also? That is, will a 50/50 mix of 7.6 pH and 6.0 pH result in water with a 6.8 pH ( (7.6+6.0) / 2.0) )?
And, a related Beersmith question...
Beersmith's "Water Profile Tool" can supposedly combine 2 known water profiles ("Base Profile" and "Dilute With") and then provide the necessary adjustments to target a 3rd profile ("Target Profile").
For a 50/50 mix of 9 gallons total, I am entering 4.5 gallons of my tap water profile ("Base Profile") and 4.5 gallons of my R/O water profile ("Dilute With"). Then, I select my "Target Profile" and hit "Match Target Profile". That results in a set of calculated additions to approximate the target profile.
Now, as a comparison to the above, I manually create a water profile for a 50/50 mix of my 2 waters (using the above-mentioned simple math). Then, using the "Water Profile Tool", I added 9 gallons of my 50/50 profile as "Base Profile", nothing for the "Dilute With" profile, selected the same "Target Profile" as before, and hit "Match Target Profile".
The resulting additions are not even close to the first method. Essentially, they're all basically 2x of the first method (I assume because the water quantity is 2x the first method). But, I don't understand why that is. I mean, ultimately, both are intended to be the same 50/50 mix of the two base waters...
In one case, I simply created a "pre-mixed" profile and in the other case, I allowed Beersmith to do that for me. Shouldn't the resulting additions be the same in both cases? I assume I'm misunderstanding something in Beersmith, but I'm not sure where my thinking went off the rails.
Thanks for any input you can provide.
I have a Ward Labs report for my tap water and I'd like to dilute it with some R/O water to tone down some of the compounds. I also have a report for the R/O water (from the provider) that I'll assume is accurate for my purposes.
I assume that calculating the compounds resulting from a mix of the two waters is just a direct, linear relationship based on the percentages of the 2 waters in the final mix, right?
For example, if water A is 50 Na and water B is 30 Na than a 50/50 mix of the two waters will be 40 Na ( (50+30)/2 ), right?
From a mathematical perspective, that makes perfect sense to me, but I wanted to make sure there's not some other chemistry-based magic going on here.
Assuming the above is correct, does that hold true for final pH values also? That is, will a 50/50 mix of 7.6 pH and 6.0 pH result in water with a 6.8 pH ( (7.6+6.0) / 2.0) )?
And, a related Beersmith question...
Beersmith's "Water Profile Tool" can supposedly combine 2 known water profiles ("Base Profile" and "Dilute With") and then provide the necessary adjustments to target a 3rd profile ("Target Profile").
For a 50/50 mix of 9 gallons total, I am entering 4.5 gallons of my tap water profile ("Base Profile") and 4.5 gallons of my R/O water profile ("Dilute With"). Then, I select my "Target Profile" and hit "Match Target Profile". That results in a set of calculated additions to approximate the target profile.
Now, as a comparison to the above, I manually create a water profile for a 50/50 mix of my 2 waters (using the above-mentioned simple math). Then, using the "Water Profile Tool", I added 9 gallons of my 50/50 profile as "Base Profile", nothing for the "Dilute With" profile, selected the same "Target Profile" as before, and hit "Match Target Profile".
The resulting additions are not even close to the first method. Essentially, they're all basically 2x of the first method (I assume because the water quantity is 2x the first method). But, I don't understand why that is. I mean, ultimately, both are intended to be the same 50/50 mix of the two base waters...
In one case, I simply created a "pre-mixed" profile and in the other case, I allowed Beersmith to do that for me. Shouldn't the resulting additions be the same in both cases? I assume I'm misunderstanding something in Beersmith, but I'm not sure where my thinking went off the rails.
Thanks for any input you can provide.
Last edited: