kidneythief
Member
- Joined
- Apr 5, 2022
- Messages
- 5
- Reaction score
- 4
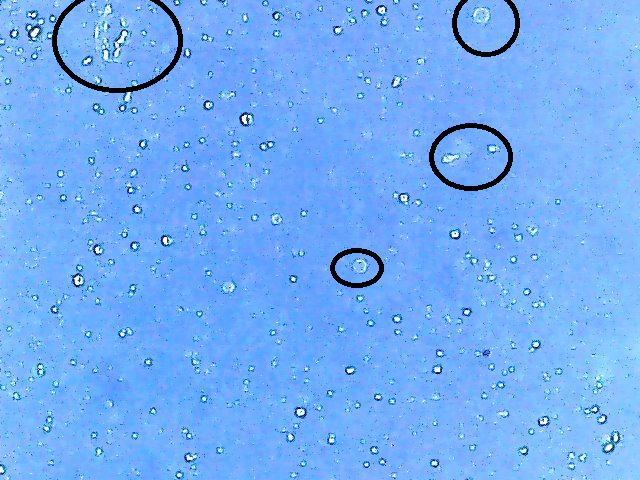
I've always wondered how many viable yeast cells there actually are in an 11.5g sachet of yeast (SafAle US-05 in this case). I am soon getting access to some basic lab equipment so I was hoping to learn how one would go about taking a cell count of a fresh, unused packet or brick of yeast.
Would I need to make a starter and take a sample from the resulting slurry or could I simply rehydrate with distilled water (using the packet recommendation of 1:10 yeast/water ratio?) and take a sample from there for the hemocytometer? Also, will methylene blue effectively stain a first generation batch of yeast cells (I've read cell walls may be thicker in first gen)?
Any information would be much appreciated! Cheers
Would I need to make a starter and take a sample from the resulting slurry or could I simply rehydrate with distilled water (using the packet recommendation of 1:10 yeast/water ratio?) and take a sample from there for the hemocytometer? Also, will methylene blue effectively stain a first generation batch of yeast cells (I've read cell walls may be thicker in first gen)?
Any information would be much appreciated! Cheers





































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)



















 )
)
