- Joined
- Feb 16, 2012
- Messages
- 4,644
- Reaction score
- 7,067
- Location
- At home, in the brewery in Maryland.
So I wandered headfirst into a rabbit hole today with the help of Google Search. The quest was for a simple answer as to methods for estimating yeast cell counts in a slurry derived from an over-built starter. That number is almost always the entering argument for any of the many yeast pitch calculators, and yet without the aid of a microscope or automatic cell counter, if the primary entering argument is off then the calculated answer will be off. Since most "ball park" estimates vary from 0.25B to 4B cells per mL, the accuracy of any calculated total cell count is pretty much useless. Don't misunderstand what I'm saying. Most of the calculators do a reasonably accurate job of giving an answer, but if the input data are wrong, then so is the output data.
Eventually, after nearly breaking the Interweb, I came across an old archived article in Brew Your Own that made a lot of sense. The writer was a graduate of Cal/Davis and well versed in brewing science. The article also is a good primer on definitions of what constitutes a slurry, while providing a simple methodology for (semi-)accurately guesstimating total yeast cells in a slurry. All you need is a graduated cylinder, a sample of slurry from a yeast starter, and the Yeast Slurry Standard Curve graph that the author compiled from data obtained from White Labs. So, ya' know, not my work and properly attributed.
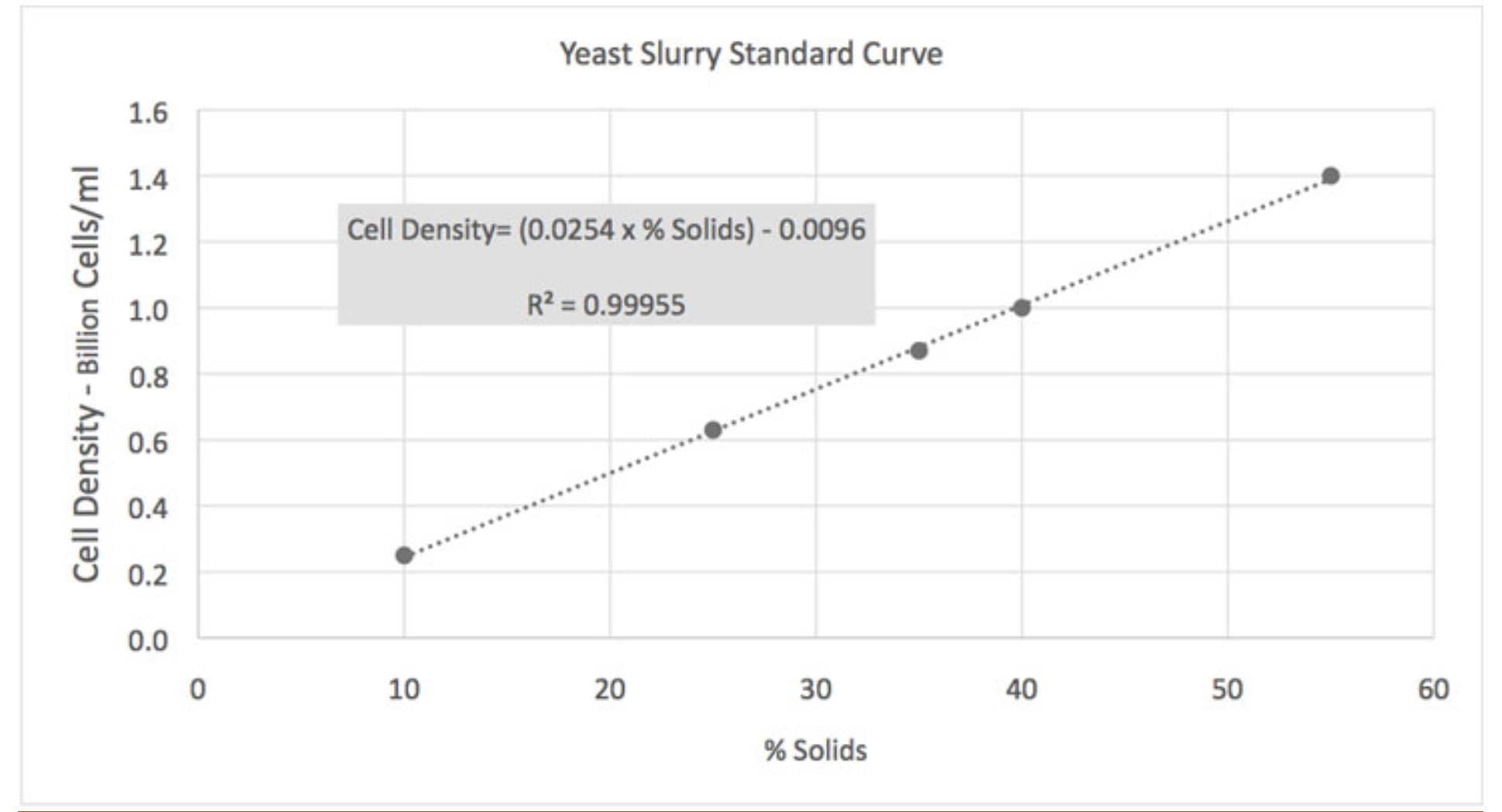
The steps are pretty straight forward. Swirl your sample to suspend all the solids (the accuracy of the process is much higher if your sample comes from a fresh starter rather than a harvest which likely has "other" solids like hops or adjuncts). Pour 100ml of the suspended slurry into the graduated cylinder. Put the cylinder in the refrigerator for 24 hours to settle the solids. The next day, measure the volume of the settled solids and convert to a percentage (i.e., 40ml settled, compacted solids = 40% of the sample). Use that value to enter the chart along the Standard Curve line. Move left parallel to the Y axis and read Cell Density - Billion Cells/ml on the X axis. Done.
Once you have that estimate of cell density from the chart, you can enter that value into one of the online calculators to derive a count of viable cells in that volume, and go from there to calculate how many steps you need to build up the total viable cell count for your brew session, based on type of beer and batch volume. The author acknowledges that cell density varies with different yeasts but the scale of the variance is not a significant factor. Cell compression is a larger factor, but due to their suspension in a liquid media the amount of compression is also considered to be a non-significant factor.
This seems like a reasonably easy and accurate method for estimating total cell count recommended for a brew session. It's certainly a few orders of magnitude more accurate than "estimating" some arbitrary value between 0.25B and 4.0B cells per ml. Are their any microbiologists out there who can attest to the methodology?
Eventually, after nearly breaking the Interweb, I came across an old archived article in Brew Your Own that made a lot of sense. The writer was a graduate of Cal/Davis and well versed in brewing science. The article also is a good primer on definitions of what constitutes a slurry, while providing a simple methodology for (semi-)accurately guesstimating total yeast cells in a slurry. All you need is a graduated cylinder, a sample of slurry from a yeast starter, and the Yeast Slurry Standard Curve graph that the author compiled from data obtained from White Labs. So, ya' know, not my work and properly attributed.

The steps are pretty straight forward. Swirl your sample to suspend all the solids (the accuracy of the process is much higher if your sample comes from a fresh starter rather than a harvest which likely has "other" solids like hops or adjuncts). Pour 100ml of the suspended slurry into the graduated cylinder. Put the cylinder in the refrigerator for 24 hours to settle the solids. The next day, measure the volume of the settled solids and convert to a percentage (i.e., 40ml settled, compacted solids = 40% of the sample). Use that value to enter the chart along the Standard Curve line. Move left parallel to the Y axis and read Cell Density - Billion Cells/ml on the X axis. Done.
Once you have that estimate of cell density from the chart, you can enter that value into one of the online calculators to derive a count of viable cells in that volume, and go from there to calculate how many steps you need to build up the total viable cell count for your brew session, based on type of beer and batch volume. The author acknowledges that cell density varies with different yeasts but the scale of the variance is not a significant factor. Cell compression is a larger factor, but due to their suspension in a liquid media the amount of compression is also considered to be a non-significant factor.
This seems like a reasonably easy and accurate method for estimating total cell count recommended for a brew session. It's certainly a few orders of magnitude more accurate than "estimating" some arbitrary value between 0.25B and 4.0B cells per ml. Are their any microbiologists out there who can attest to the methodology?
Last edited by a moderator:


























![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)































