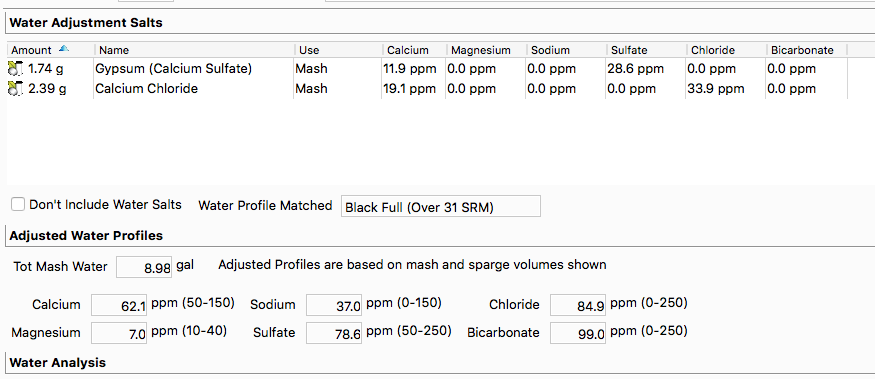
Calcium Chloride continuously absorbs water from the air each time it is exposed to it. It appears that Bru'n Water (free edition) presumes that CaCl2 is completely water free (or anhydrous), whereby even right out of a freshly opened bottle or bag it is usually only at best going to be about 94-96% pure (based upon my two tests of it) as opposed to 100% pure as presumed by BW_free. Most other software presumes that CaCl2 is most typically going to be found in it's dihydrate state, or at about 75.5% purity. Albeit that the latter may be a better outright guess to some extent, none of this is technically "correct". CaCl2 continues absorbing moisture until at somewhere around the heptahydrate state it finally turns into a liquid goo. But before that it pretty much looks outwardly the same (as dry prills). CaCl2 does not jump from state to state, but can exist in a blend of states. As I recall it takes very high heat to drive it back towards the anhydride state. Look it up and don't quote me on this, but it will likely be found to require somewhere around 425 degrees F. plus time. But even if you dry it to nearly 100% purity, by the time it has cooled sufficiently enough to return it to storage it will likely already be only about 95% pure. Due to contaminants it is never actually going to be 100% pure. I believe that AJ discovered the major contaminant to be calcium carbonate.
My always free and complete 'Mash Made Easy' spreadsheet permits you to select any percentage of purity for your CaCl2, or to use it as a liquid solution at any measured specific gravity. As a liquid solution it is much more stable. If your solution drops out a white precipitate over time that is likely calcium carbonate. The absorption of CO2 from exposure to air may over time drop out additional calcium carbonate, plus there is evaporation, so even a liquid solution is not fully stable. Recheck the specific gravity occasionally (perhaps twice a year) whereby to correct for this.





![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)