Firstly AJ, may I commend and thank you for the detailed explanation of the theory and priciples involved.
You are certainly most welcome but I am as interested in resolving this discrepancy as you are. I'll also note that the new spreadsheet I have been putting together breaks the problem down in such a way that it furnishes exactly the sorts of information I have been posting and is thus perfect for this problem.
I was able to follow and understand all you wrote, but feel it may work better when brewing pale beers with low mineral levels such as Pilsner.
There is one aspect to this which may indeed make it work better for low mineral beers and that is the question of how many protons are released by a mEq of calcium. Kohlbach's well known finding is that 3.5 mEq of calcium have released 1 mEq of protons by the time knockout is reached and that magnesium releases half as many. But we aren't concerned about knockout here but at the beginning of the mash. Also Kolbach's result presumably applies to pre WWII German brewing practices and this may or may not be applicable to British brewing in 2018.
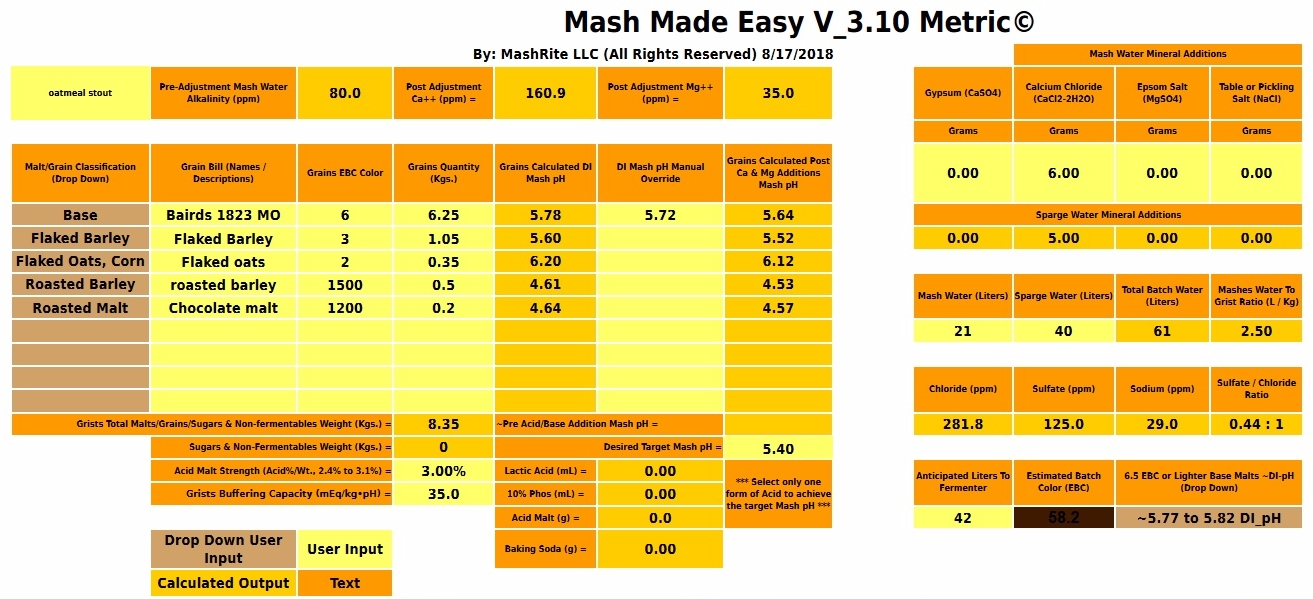
If you tell me you have calcium at 83 mg/L (4.15 mEq/L) how much H+ should I assign to that? Following Kolbach that would be 4.15/3.5 = 1.2 mEq/L. At 2.25 mash thickness that's 2.7 mEq/kg which is appreciable compared to the 9.69 mEq/kg deficit for the malt. But if we assume that only half of these protons are released in the mash tun (which is what I asssume) then the proton surfeit from calcium is 1.35 mEq/kg there. Still not unappreciable. With your mash buffering estimated at 0.021 pH/mEq•kg the pH shift caused by calcium would be around 0.03. That doesn't go very far towards explaining a discrepancy of 0.2 pH but it isn't something we can brush off either. With softer water there is less calcium and thus less potential error ascribable to mis-modeling of it.
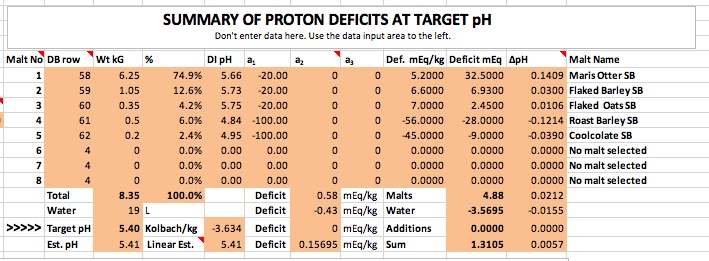
The rest of the problem depends entirely on the modeling of the malt. The range of dI pH and buffering for the colored malts is much wider than for the pale malts so I guess we could accord them higher potential for error based on that. But the black malts are more tightly grouped than the base malts or the crystal/caramels so I don't really see much in this suggestion. But I do recognize that fed bad malt data this calculator, as will any other, will return bad predictions.
I expected the predicted calcium level to be significant, but not of such magnitude and perhaps makes this the crux of the matter. It was possible that I gave mineral levels that were marginally inaccurate, but it would be impossible for all or any to have this order of error. If 40 times more calcium be needed to correct the difference between my findings and the calculated pH, then wouldn't reducing alkalinity in accord to Kolbach's findings to something like 8 ppm similarly account for the discrepancy?
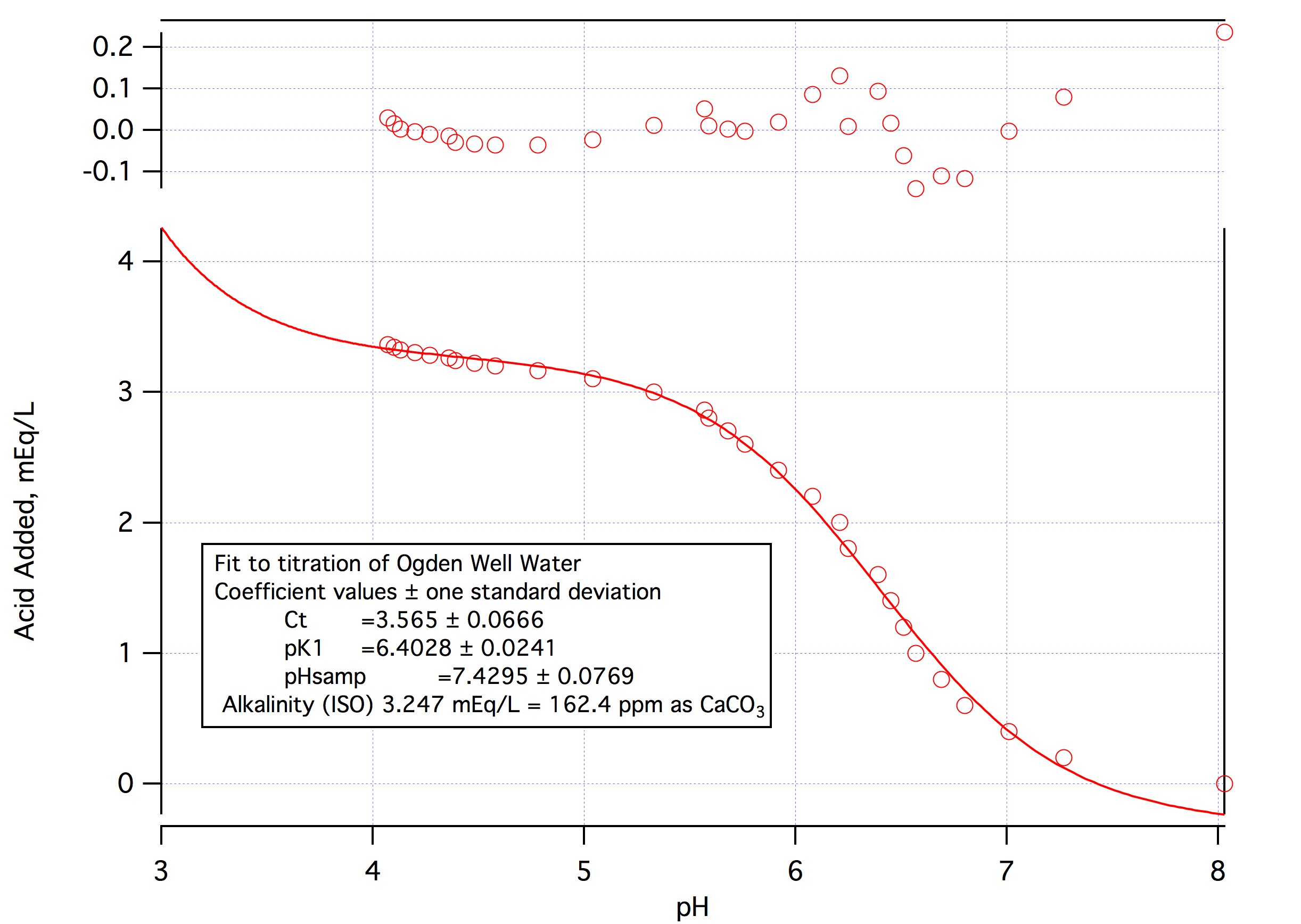
I only calculated the calcium correction to illustrate that blaming incorrect reporting of calcium as the source of the discrepancy would be absurd. Taking out the alkalinity altogether would only change the estimated mash pH from 5.64 to 5.58.
This would be equally ridiculous to me as I regularly mash all pale malt grists in liquor with catons of similar amounts as the stout brew and alkalinity around 20 ppm to achieve pH 5.2 to 5.3.
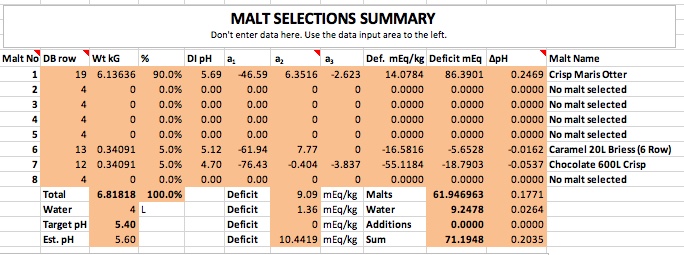
This says that you are going to mix say 80% base malt with a DI pH of 5.6 or above with 20% of some crystal or caramel malt with a pH of around 5.1 - 5.4 (presumably it would be a reasonably light malt) and realize a pH of 5.2. That just doesn't make sense unless we ascribe a pH lowering effect to calcium that simply isn't realistic. One of the things I love about this new spread sheet it that it is easy to answer the question "How unrealistic?" The answer is that rather than the Kolbach factor being 7 (half in mash tun, half in kettle) it would have to be 0.64. That means that instead of requiring 7 mEq of calcium to produce a mEq of protons each mEq of Ca++ would produce 1.55 mEq of protons. Impossible.
(yes, Were alkalinity to be that order for the stout, mash pH would almost certainly be less than 5.
But calculation and my experience say that it will be around 5.5 - 6.0. That's what I get when I put the numbers in the calculator and that's what I measure when I make beers like this.
When home brewing started in UK fifty five years ago, the expertise was with commercial brewers. Wine making at home was legal and that community supplied the enquiring homebrewers with advice that produced a great many dreadful beers. In his 1974 book,
Dave Line
His "The Big Book of Brewing" and Bravery's "Home Brewing Without Failures" are what got me started in this business.
....advised how to brew an all grain version of Guinness made using 70% pale, 20% flaked barley and 10% roast barley, not magnitudes away from the brew under discussion. That recipe is still popular in UK to this day.
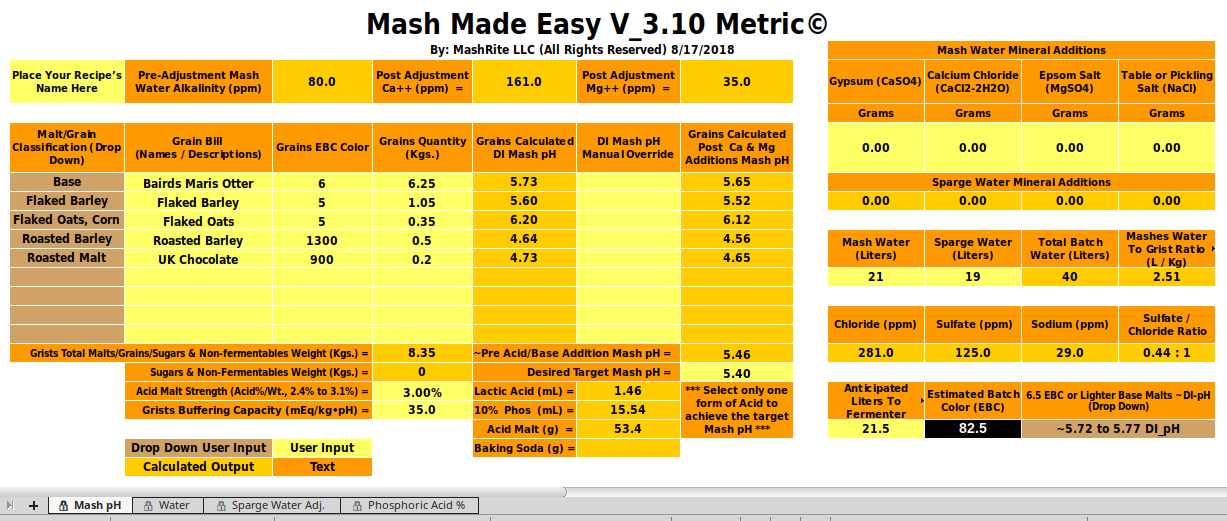
When I make stout that's the recipe I use (though I think I got it from Michael Lewis's monograph rather than Dave Line. I've done it at least several times and I always get, with water of alkalinity of about 80 and hardness of about 110 (total) a mash pH of 5.5 - 5.6. Why are you getting 5.4? No answer proposed answer related to malt or minerals seems to adequately explain and that gain puts the limelight back on the pH measurements. But you assure us that you have properly calibrated the meter. Have you run a stability check on it?
Then I looked at the picture. The buffers! Your pH meter is only as accurate as the buffers you calibrate it with. I don't see any "NIST traceable" markings on those bottles. There have been reports on this forum by people who have bought buffers for pH meter calibration only to find that their actual pH's were quite a bit off from the package labeling. I am not suggesting that this
is the case here but that
it might be. Let's face it, I'm grasping at straws at this point. Bad buffers may be an unlikely explanation but at least it's one I can comprehend.
I'd struggled with water to that time, but brewed 5 (imperial) gallons as specified with a teaspoon of calcium salts to both the mash and kettle. My water at that time had roughly 100ppm calcium and 250 ppm alkalinity as CaCO3, but the resulting beer was revelationary for me, that water could make a reasonably good stout.
I have never resorted to using lactic acid or sauermalz in a stout or porter. For such beers my supply water treated with mineral acid to...
. A proton is a proton. It doesn't matter whether they come from an organic or inorganic acid (as long as the weak nature of the organic acids is taken into consideration). I only used lactic because it it the first acid button on my spreadsheet. In terms of pH control you should be looking at the mEq only. But of course the anion involves can have a big influence on the way the beer tastes. For the record, I don't use acids in a stout. As long as pH < 5.6 I generally call that close enough and certainly the results have been good in terms of a tasty product at the 5.6 level.
reduce alkalinity to between 70 and 100 ppm as CaCO3 with various amounts of additional calcium salts can achieve any and every acceptable mash pH.
If you've got 1.4 - 2 mEq/L of alkalinity to dispose of then you'll need 7 times that much calcium per liter i.e. 9.8 - 15 mEq/L. That's assuming the half/half split between mash tun and kettle for the Kolbach reaction. If we assume it all takes place in the mashtun that means the requirement could be onl 4.9 mEq at the low end. As we really haven't a clue as to how it divvies itself up the possible range of calcium requirement for pH control would be 4.9 - 15 mEq/L or 186 - 300 mg/L calcium. To my way of thinking that's a lot of calcium! 245 - 750 ppm calcium hardness.
It would be were it true but there was a typo there. It should have read 5.5 -5.6.
Before I could accurately control alkalinity although able to measure pH, all pale malt beers could have a mash pH slightly over 6.
That would take a base malt with a pretty high DI pH and huge alkalinity. Muntons MO and 400 ppm as CaCO3 would do it.
However, I can't ever remember making Dave Lines Guinness with a mash pH that high, even with alkalinity in excess of 200 ppm as CaCO3.
No. Even 200 ppm alkalinityu should only get you to about 5.75.
I wonder, is your alkalinity achieved by adding sodium (bi)carbonate. If so, might it react differently than alkalinty formed from calcium and magnesium?
It's moot but I'll comment that bicarbonate is bicarbonate in terms of its alkalinity whether it is paired with sodium, calcium or magnesium. Same for carbonate (except for the solubility issue with calcium carbonate).























![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)