My thanks to both.
Grist:-
Bairds 1823 MO 6 EBC
Crisp Flaked Barley 3 EBC
Crisp Flaked Oats 2 EBC
Bairds Roasted Barley 1300 EBC
Bairds Chocolate Malt 950 EBC
However, according to the data on Bairds website Roast Barley can be as high as 1500 EBC and Chocolate Malt up to 1100 EBC.
I'll point out again that malt color just isn't a very good way of determining malt acidity. I just went back and looked at Kai Troester's data. He found r = 0.72 which means that only half the variation in acidity he observed is attributable to color. The rest is random! And that's for base malts. For the roast malts he found no correlation. They all have more or less the same acidity irrespective of color. I'd guess that once you have roasted a grain to a certain pint all the acid that is going to be produced has been produced and further roasting only serves to further carbonize.
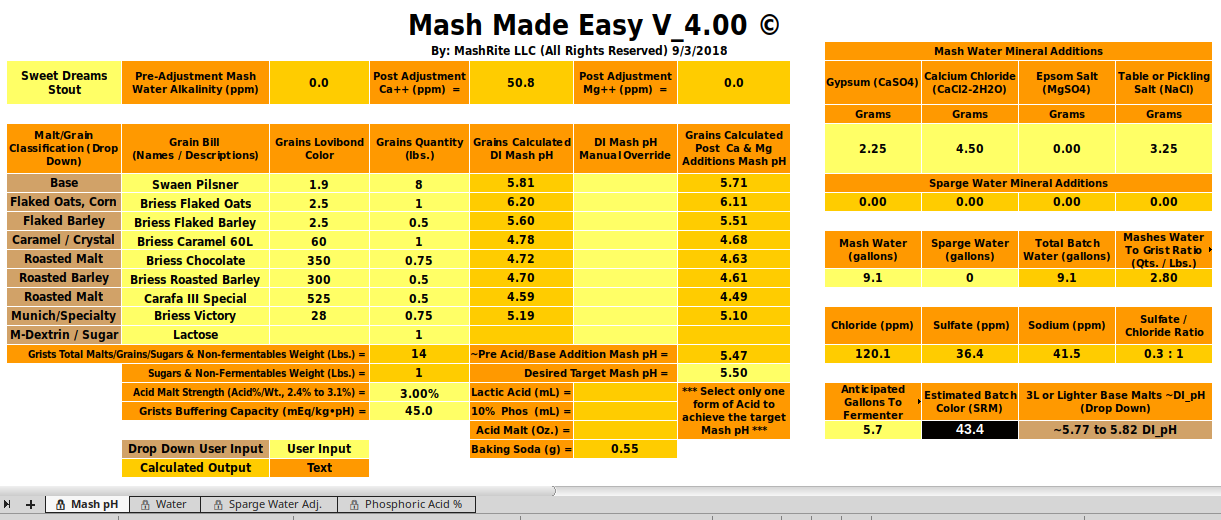
I noted in No. 141 that having so much calcium present effectively nullifies the alkalinity and, of course, that leads to lower mash pH. In fact, as the table shows, this water is effectively an acid with respect to pH 5.4.
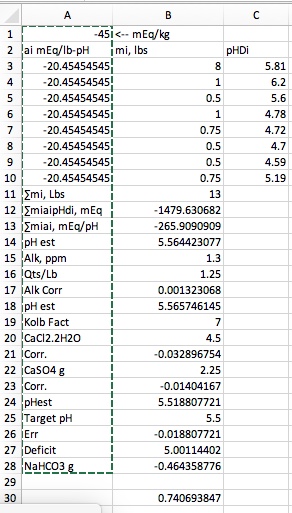
I predicted 5.56 for the Dave Line stout. For the recipe in the quote I ran again using the models that silver has posted based on cire's measurements of pHDI for the Maris Otter and the Chocolate. One concept that I don't seem to be able to get across is the concept of buffering nor do I seem to be able to get people to understand that the acidity (or the alkalinity) of a malt depend on the malt's pHDI and its buffering and the mash pH. A pHDI measurement of pHDI tells me more than the fact that the malt is MO but I still need the buffering value in order to be able to calculate acidity for a particular mash pH. The good news is that base malts seem to have buffering capacities of about -40 while darker malts seem to run larger (magnitude) numbers. I can therefore guess at what the buffering of a malt might be from the malt type. Doing that here's the summary:
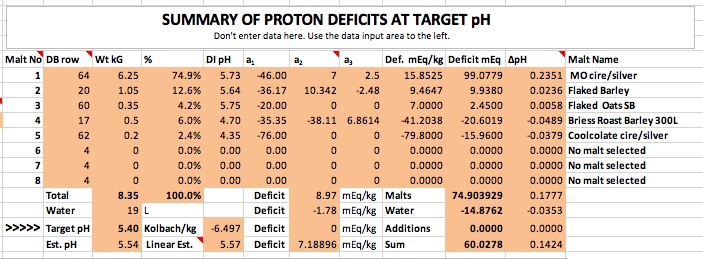
As you can see I'm getting closer but still can't get down to 5.4 and for the same reason. The dark malts simply don't have enough acidity to overcome the alkalinity of the base malts even though the base malts in this run are less alkaline than the ones I've used previously. I really think the discrepancy between what I calculate and what the Gen I guys are calculating has to do with the way they handle buffering. But I can't get an explanation as to how they do that that I can understand.
Note that the Est. pH field represents the pH obtained by solving the non linear equation for deficit vs pH when the deficit is set to 0. The number in the Linear Est field is an estimate based on the Riffe equation which ignores non linearity as Gen I programs do. I thought this might have something to do with the discrepancy and was thus a bit surprised to find that the linear approximation result is higher than the full solution.
Last edited:





![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)