First off, let me qualify my statement about the necessity of a pressure cooker by saying that I am a sterility expert. I've spend the last 17 years validating sterilization processes for the medical device industry. While most of my work is with gas, I have validated many steam sterilizers all over the globe and have peer-reviewed published literature regarding microbial lethality. The standard autoclave runs at 121°C for 15 minutes at X psi depending on the sterilizer. The pressure allows the superheating of water to temperatures above boiling. Water boils at 100°C--give or take, and still sterilizersjust slightly slower.
Now, let's talk about microbial lethality. Heat is a very good vehicle for rendering bugs dead. At 121°C, you can kill one (1) log of the absolute most resistant spore formers in about 2.5 minutes (it's decimal reduction value or D-value). Let's say this decimal reduction value is 2.5 mins. You will reduce 1,000,000 bugs (6 logs) in 2.5 mins x 6 = 15 mins.
Now, at 100°C, you are still going to get kill, it's just the D-value is going to be longer. Most everyday bugs are so much easier to kill than the resistant spores used to validate steam sterilizers. In fact, the typical D-value will probably be less than 1 minute at 100°C. That means that if you had 1,000,000 bugs in your vial, all it would take is boiling it for 6 mins at 100°C and everything would be dead. Now, run it for 15 minutes. Nothing is going to survive.
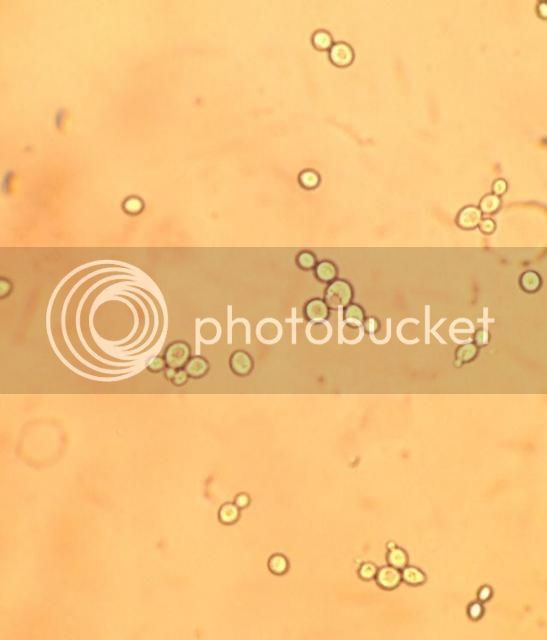
Keep in mind that with yeast slanting, you have already boiled your wort and added gelatin. Even with a non-sterile vial, you are probably starting with a handful of bugs at the most--maybe 100-1000 if your vials were dirty. Three (3) mins at 100°C will kill them. (Keep in mind that the temperature of the vial will be slower to heat than the water in the pot; the glass vial will act as a thermal barrier and slow the heat up time. You could add additional heat time, but frankly I wouldn't bother.)
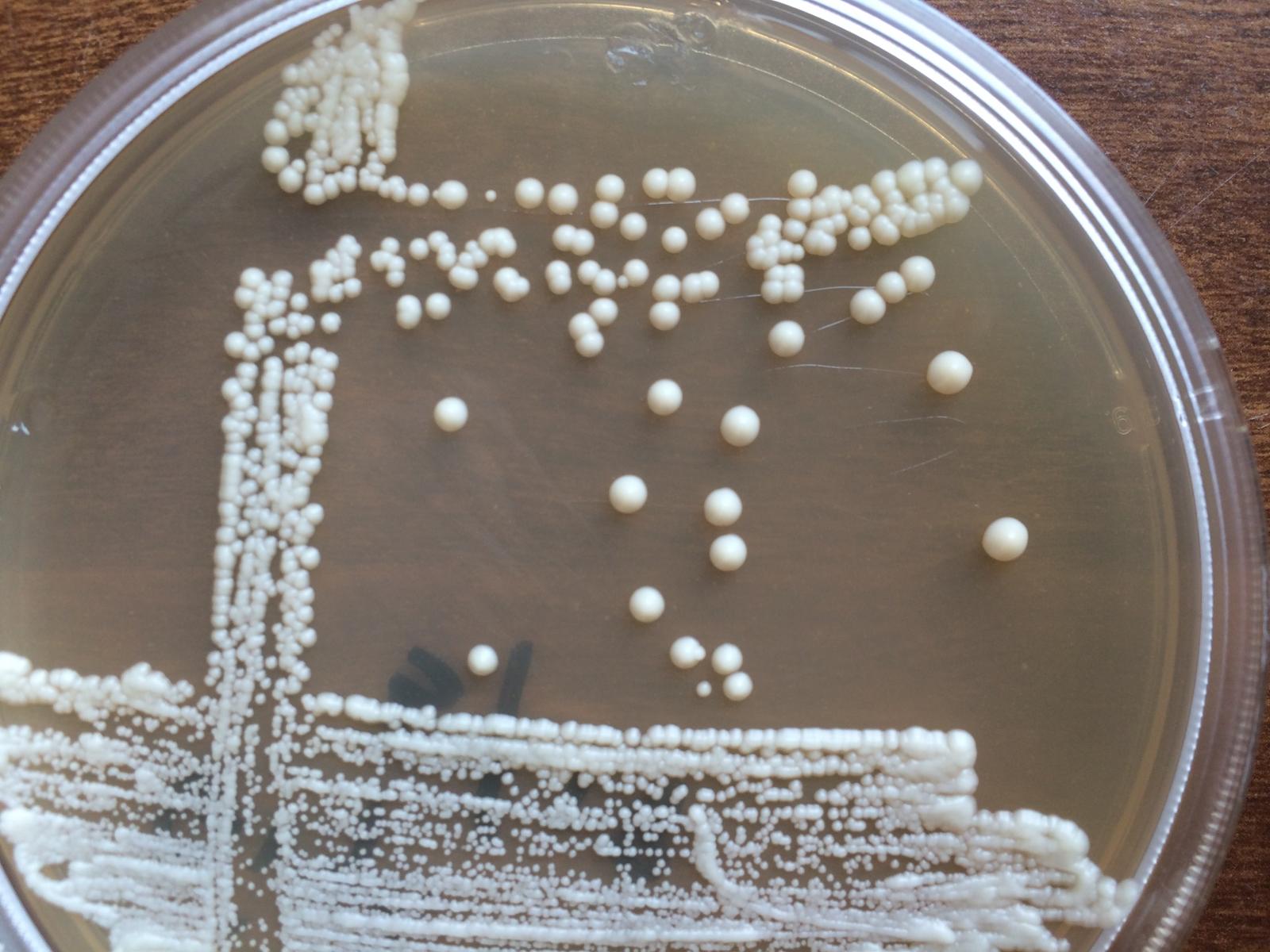
You can verify sterility success 100% through inspection, that is leave your vials out for 14-days at room temperature (capped of course), and if they are free of bugs, you have a sterile product. I only use a pan, a veggie steamer to keep it off the bottom and s few inches of water.
Check out my webpage. I have a 9-step process with imagesthe whole process is less than an hour.
http://www.acapellabrewing.com/project/diy-yeast-slants/
Cheers.













![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)