Brew_Meister_General
Well-Known Member
- Joined
- Aug 27, 2015
- Messages
- 256
- Reaction score
- 14
Well I look forward to the day when I'm as knowledgeable as the guys who have participated in this thread - bring it on.
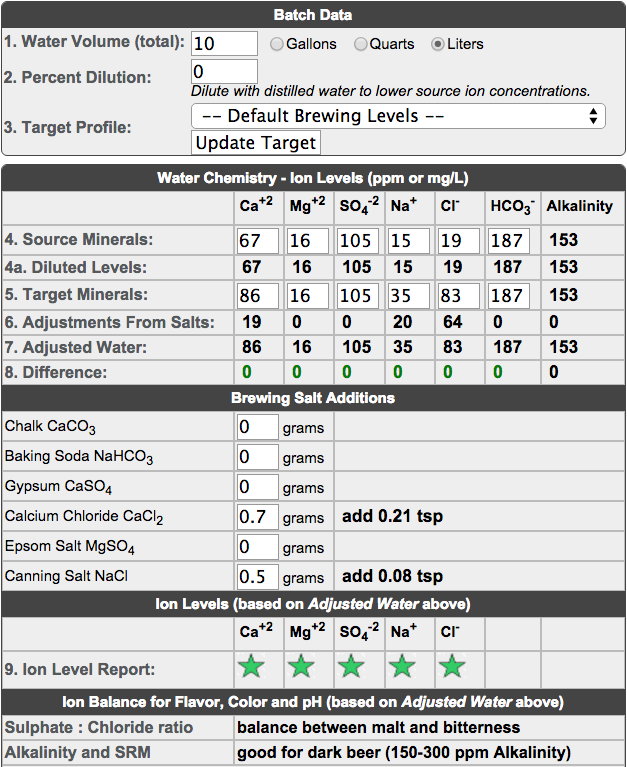
I got more comprehensive report from my water company... http://www.ywonline.co.uk/web/WQZ.nsf/0/08F2F7D0B5D90B47802574FD003904A0/$file/Northallerton%202004%20WSZ.pdf
Seems my water is pretty bang on for bitters, porters and stouts.
So to summaries...
Calcium 67 (50 - 82)
Magnesium 16 (10 - 20)
Sodium 15 (12 - 17)
Sulphate 105 (77 - 125)
Chloride 19 ( 17 - 22)
pH 7.3 (7.1 - 8.3)
Yikes! Your sulphate is high which means it would be ideal for brewing IPAs and other bitter beers. That being said if you increase your chloride, which is very low in comparison, to 85 or 90 say (but away from 100) then that will produce a balance between sweetness and bitterness and also allow you to increase your calcium and sodium in the process using calcium chloride and sea salt.
So using the Basic Water Chemistry calculator on Brewer's Friend this is what your salt additions might look like per 10l of water:
I've also read never to use table salt as it contains iodine which sea salt and pickling salt doesn't.
And wow that is a huge swing in bicarbonate! I haven't come across that before, I'd be curious to find out why now, maybe its seasonal? But every problem has a solution! It'd probably to start a thread and get some expert feedback on your water chemistry.















































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)











