Nodak_Brewer
Well-Known Member
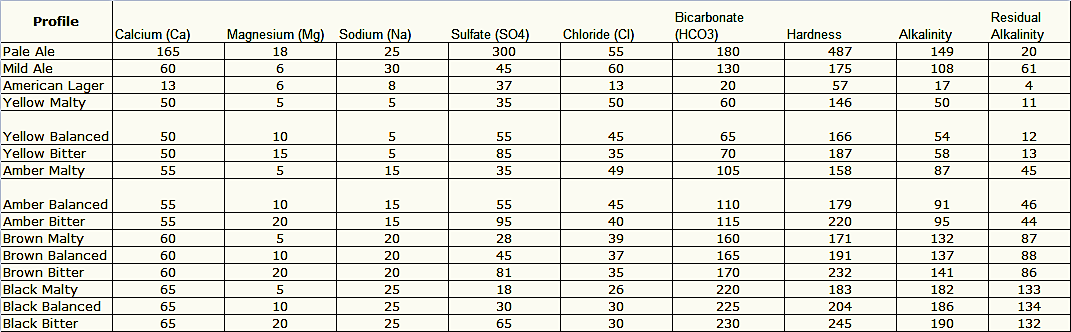
I'm not ready to delve into advanced brew water science, but I'd like to alter my water a little more precisely for lighter brews, more specifically German beers like hefeweizen, kolsch, etc. My water is very hard and alkaline, and in the past I just split it 50/50 with distilled and tap water, treated entirely with K-meta.
Does anyone else have hard/alkaline water and use a specific ratio of RO or distilled water to tap water, and do you treat all of it or just the stuff from the tap?
Does anyone else have hard/alkaline water and use a specific ratio of RO or distilled water to tap water, and do you treat all of it or just the stuff from the tap?