You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Am I calculating my ph right?
- Thread starter MPBeer
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
You don't want to use chalk. Ever - well hardly ever. Be suspicious of any calculator or spreadsheet which allows chalk to be entered unless its use is heavily caveated in the instructions or by lighting up red or somehow otherwise warning you when any is entered.
Last edited:
tgodsill
Active Member
- Joined
- Dec 16, 2017
- Messages
- 31
- Reaction score
- 9
+1 on what AJ said. If you are using the new BeerSmith3 software there is even a selection to omit chalk from all brewing salt calculations. Also, if you are still able to adjust your recipe you might want to think about going more simple with the grain bill. 10 different grains is quite a lot! Good luck!
Smellyglove
Well-Known Member
- Joined
- May 17, 2013
- Messages
- 2,807
- Reaction score
- 807
There's nothing wrong in using ten different grains in a imp stout..
oldwhiskers
Well-Known Member
- Joined
- Dec 30, 2013
- Messages
- 55
- Reaction score
- 16
That looks like Brewers Friend, if it is, also check the pH using beer color from receipe builder. I did a batch last week and looking at it afterwards I got two different pH calculations. The one calculated from beer color was closer to what I measured.
MPBeer
Well-Known Member
- Joined
- Dec 12, 2017
- Messages
- 98
- Reaction score
- 10
That looks like Brewers Friend, if it is, also check the pH using beer color from receipe builder. I did a batch last week and looking at it afterwards I got two different pH calculations. The one calculated from beer color was closer to what I measured.
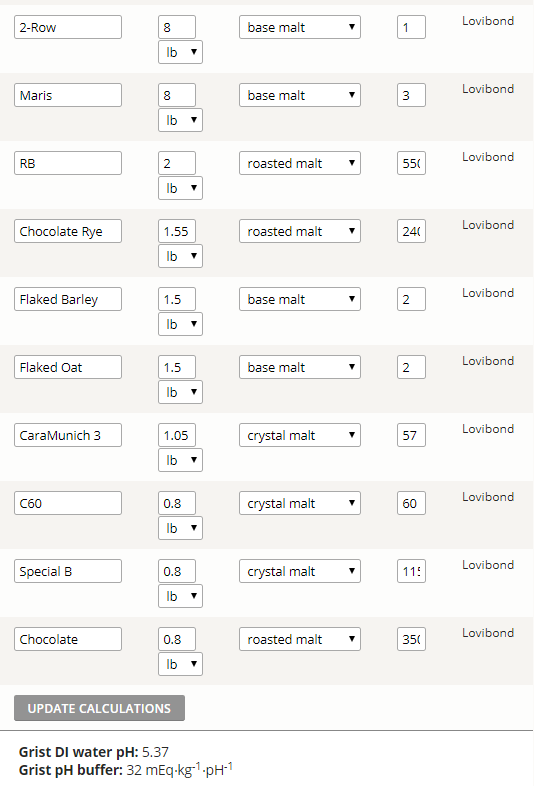
Yeah I input 20~25% of roasted malt and I got 4.8~ ph... there are a huge difference and it seems like inputting hard numbers are more accurate so I'm not sure which to trust

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$172.35
2 Inch Tri Clamp Keg Manifold With Ball Lock Posts, Pressure Gauge, PRV (0-30 PSI) – Homebrew, Fermentation, Kegging System
wuhanshijiayangzhiyimaoyiyouxiangongsi

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop
![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)
$6.95 ($17.38 / Ounce)
$7.47 ($18.68 / Ounce)
Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]
Hobby Homebrew

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive

$33.95
Five Star - 6022b_ - Star San - 32 Ounce - High Foaming Sanitizer
Bridgeview Beer and Wine Supply

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Gas MFL)
Guangshui Weilu You Trading Co., Ltd
MPBeer
Well-Known Member
- Joined
- Dec 12, 2017
- Messages
- 98
- Reaction score
- 10
+1 on what AJ said. If you are using the new BeerSmith3 software there is even a selection to omit chalk from all brewing salt calculations. Also, if you are still able to adjust your recipe you might want to think about going more simple with the grain bill. 10 different grains is quite a lot! Good luck!
I always get so greedy when I make an stout lol. I just downloaded BerrSmith3 and seems like the ph is pretty similar. I'll go with no salts added and see if the ph was too low! Thanks!
oldwhiskers
Well-Known Member
- Joined
- Dec 30, 2013
- Messages
- 55
- Reaction score
- 16
Yeah I input 20~25% of roasted malt and I got 4.8~ ph... there are a huge difference and it seems like inputting hard numbers are more accurate so I'm not sure which to trust
I downloaded MashMadeEasy from the Brewing Software forum and plan on using it tomorrow for a brew. It doesn't take too long to figure it out. I used it to check the batch I did last weekend with BF and I got close to the pH I actually observed.
North_of_60
Well-Known Member
You don't want to use chalk. Ever - well hardly ever. Be suspicious of any calculator or spreadsheet which allows chalk to be entered unless its use is heavily caveated in the instructions or by lighting up red or somehow otherwise warning you when any is entered.
Why don’t you want to add chalk? What is the negative affect? Why do most calculators have it as an addition?
I just started using water additives using BeerSmith and for 8 gallons of mash water it calls 1.8g chalk along with 7g gypsum, 1.9g calcium chloride.
The acids in the typical mash are not strong enough to quickly dissolve chalk. There is a small amount of strong acids in the mash and a chalk addition will raise a typical mash pH by about 0.1 units, but additional chalk additions won't increase the mash pH any further. So chalk is not a good alternative for raising mash alkalinity and pH. Better options for raising mashing water alkalinity and mash pH are baking soda and lime.
The problem with chalk is that, under normal conditions i.e. where you add it to mash or water (which is effectively the same as adding it to mash as it doesn't dissolve in water), it reacts so slowly that you do not get the proton absorbing effect which you are depending on it to provide when you need it. Thus mash pH is not raised as much as you hope it would be. Much of the chalk you add to mash or water will thus wind up sitting on the grains after sparging but some will make it through into the kettle and into the fermenter where it will continue to react thus raising pH during parts of the process where you want it to be falling. You may argue that this is beneficial as it is finally doing what you added it to do but presumably you added it to control pH in the mash tun and you won't get that effect from it.
There are work-arounds such as dissolving it in acid but you need to be careful that you don't acidify below pH 8.4 (at which pH all the carbonate has been converted to bicarbonate which does react rapidly enough to do what you want to do. One of the acids proposed for this purpose is carbonic acid. To use it the CaCO3 is placed in a pet bottle with water and CO2 injected to raise the pressure to the point where enough H2CO3 is dissolved to, in turn, dissolve the CaCO3. Again the goal is to get the CO3-- converted to HCO3- and the tricky part is that as soon as the pressure is released the HCO3- will convert back to CO3-- and re-precipitate as microcrystals you cannot see and so are lulled into thinking you have dissolved the chalk. And some of is still dissolved. But how much? The problem is that you won't really be able to tell exactly what is going on with this method and you will wind up with CO3-- going forward.
The problem here is a common one in home brewing. Someone who knows enough chemistry to know that CO3-- is basic reasons that it should be able to absorb protons and thus compensate for the use of acidic malts. He doesn't experiment and he doesn't know enough about chemistry to appreciate the kinnetics. He publishes books, nomographs, spreadheets, articles in Zymurgy... and others imitate him and it becomes one of the foundations of home brewing lore that you add chalk to dark beer. Once so established these foundations are hard to eradicate.
If you need to absorb protons (and as has been noted in another thread in this forum that you really shouldn't) you need to use a faster acting base. The obvious one is Sodium Bicarbonate which a lot of people shun because of sodium phobia and, of course, if your source water is rich in sodium this can be a problem. Another base is Ca(OH)2 but I am a little wary of that because I have seen it, in the laboratory, ehibit some strange kinnetics which I don't fully understand at this point. Another candidate is Na2CO3. I'm a little wary of it too because while I suspect that the slow kinnetics with CaCO3 relate to the presence of the Ca++ ion I am not sure that using the much more soluble sodium salt removes the CO3-- problem. It seems it should though as I suspect the low rate constant applies to CaCO3 + H2O --> Ca++ + CO3-- + H2O and not to CO3-- + H+ --> HCO3-. Note, also that your water may have a fair amount of calcium in it before adjustment. Also whilst NaHCO3 gives you proton absorbing capacity of 0.9 mEq per mmol added sodium Na2CO3 only gives 0.95 which isn't much of an improvement if sodium is a problem. NaOH gives 1 mEq/mmol as does KOH (of, respectively, Na and K) but they seem to be rarely used by home brewers.
Thus I'd say NaHCO3 is your best bet if you can tolerate the sodium and if not, Ca(OH)2.
There are work-arounds such as dissolving it in acid but you need to be careful that you don't acidify below pH 8.4 (at which pH all the carbonate has been converted to bicarbonate which does react rapidly enough to do what you want to do. One of the acids proposed for this purpose is carbonic acid. To use it the CaCO3 is placed in a pet bottle with water and CO2 injected to raise the pressure to the point where enough H2CO3 is dissolved to, in turn, dissolve the CaCO3. Again the goal is to get the CO3-- converted to HCO3- and the tricky part is that as soon as the pressure is released the HCO3- will convert back to CO3-- and re-precipitate as microcrystals you cannot see and so are lulled into thinking you have dissolved the chalk. And some of is still dissolved. But how much? The problem is that you won't really be able to tell exactly what is going on with this method and you will wind up with CO3-- going forward.
The problem here is a common one in home brewing. Someone who knows enough chemistry to know that CO3-- is basic reasons that it should be able to absorb protons and thus compensate for the use of acidic malts. He doesn't experiment and he doesn't know enough about chemistry to appreciate the kinnetics. He publishes books, nomographs, spreadheets, articles in Zymurgy... and others imitate him and it becomes one of the foundations of home brewing lore that you add chalk to dark beer. Once so established these foundations are hard to eradicate.
If you need to absorb protons (and as has been noted in another thread in this forum that you really shouldn't) you need to use a faster acting base. The obvious one is Sodium Bicarbonate which a lot of people shun because of sodium phobia and, of course, if your source water is rich in sodium this can be a problem. Another base is Ca(OH)2 but I am a little wary of that because I have seen it, in the laboratory, ehibit some strange kinnetics which I don't fully understand at this point. Another candidate is Na2CO3. I'm a little wary of it too because while I suspect that the slow kinnetics with CaCO3 relate to the presence of the Ca++ ion I am not sure that using the much more soluble sodium salt removes the CO3-- problem. It seems it should though as I suspect the low rate constant applies to CaCO3 + H2O --> Ca++ + CO3-- + H2O and not to CO3-- + H+ --> HCO3-. Note, also that your water may have a fair amount of calcium in it before adjustment. Also whilst NaHCO3 gives you proton absorbing capacity of 0.9 mEq per mmol added sodium Na2CO3 only gives 0.95 which isn't much of an improvement if sodium is a problem. NaOH gives 1 mEq/mmol as does KOH (of, respectively, Na and K) but they seem to be rarely used by home brewers.
Thus I'd say NaHCO3 is your best bet if you can tolerate the sodium and if not, Ca(OH)2.
Last edited:
There aren't any strong acids in mash but there are acids that are stonger than bicarbonic acid and so they will give their protons up to CO3-- - eventually. The problem is in the time it takes. Rate coefficents in higher order systems usually depend on concentration so that it isn't that the acids aren't strong enough, it's that there isn't enough of them. Add a little hydrochloric acid to a suspension of chalk and you will see gas bubbles evolve and the pH will fall back dramatically and the suspension may even turn clear. Then, over time, the pH will creep back up again as the suspended invisible CaCO3 microcrystals dissolve and protons are absorbed. This will continue over at least 24 hours.The acids in the typical mash are not strong enough to quickly dissolve chalk. There is a small amount of strong acids in the mash...
Yes, sorry. There aren't Strong acids in a mash. Stronger is the proper term since Strong has a specific meaning in chemistry.
North_of_60
Well-Known Member
Should I substitute baking soda for chalk? If so, at what ratio?
Should I substitute baking soda for chalk? If so, at what ratio?
Baking soda is a very useful addition when brewing dark beer styles with RO or distilled water. Sure, it will boost the sodium level in the mashing water, but the eventual dilution with baking soda-free sparging water means that the sodium level in the kettle isn't so bad.
The dose is determined by using a better calculator that includes baking soda as a possible addition.
Yes, if you can live with the sodium.Should I substitute baking soda for chalk? If so, at what ratio?
The ratio? I'm going to have to go through some stuff here to get the answer. If you are interested in how this stuff works you will learn something.
In transitioning from NaHCO3 powder to HCO3- ion in a mash at pH 5.3 a mole of the powder will absorb 0.92 mEq of protons. If mash pH is 5.4 it will absorb 0.905 and if it is 5.5 it will absorb 0.88. In transitioning from the powder to HCO3- ion in a mash a mole of CaCO3 will absorb 1.92 mEq protons if the mash pH is 5.3. At 5.4 it will absorb 1.905 and at 5.5, 1.88. Thus if a brewing program has correctly calculated the proton surfeit for a mash (and this is not likely) and is aware that CaCO3's proton absorption potential is about 1.9 mEq/mmol it will give you the stoichiometrically correct amount of CaCO3 to absorb those protons regardless of the fact that that many protons will not be absorbed if CaCO3 is added to a mash. Then to get the number of moles of CaCO3 that corresponds to you divide the recommended grams of CaCO3 by the molecular weight of CaCO3 which is, conveniently, 100. We have just shown that a mole of HCO3- absorbs 0.9/1.9 = 0.473 times as many protons as a mole of CaCO3 thus we will need to multiply the recommended moles of CaCO3 by 1.9/0.9 to get the moles of NaHCO3 required. Up to this point if we had a recommendation of grams of CaCO3 we would conclude that we would need (x/100)*(1.9/0.9) moles of NaHCO3. All that remains is to multiply by the molecular weight of NaHCO3 which is 84 to get the required weight of NaHCO3. Putting all this together we get
grams_NaHCO3 = (84/100)*(1.9/0.9)*grams_CaCO3 = 1.773*grams_CaCO3.
The problem with this is that most of the programs are unaware that bicarbonate only absorbs 0.9 mEq/mmol and carbonate 1.9 mEq/mmol (at pH 5.4). They thinK the numbers are, respectively, 1 and 2. This would make the ratio (84/100)*(2/1) = 1.68 - not a whole lot different from 1.77 but different nevertheless. There is at least one program that I am pretty sure handles bicarbonate properly in this regard. As Martin is monitoring this thread he can let you know whether Bru'n water has been fixed. As for any of the others I just don't know.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
A.J., for a given mash pH target can this simple slope formula be applied to compute baking sodas molar NaHCO3 to HCO3- absorbance factor over the typical mash pH target range of 5.2 to 5.6:
mEq_absorbance_factor = -0.2 * target_pH+1.98
Then I presume that it simply becomes a matter of:
(ideal moles of required NaHCO3) / mEq_absorbance_factor = actual moles NaHCO3 required to be added
Does this seem to get things into a better ballpark?
mEq_absorbance_factor = -0.2 * target_pH+1.98
Then I presume that it simply becomes a matter of:
(ideal moles of required NaHCO3) / mEq_absorbance_factor = actual moles NaHCO3 required to be added
Does this seem to get things into a better ballpark?
Here's a plot
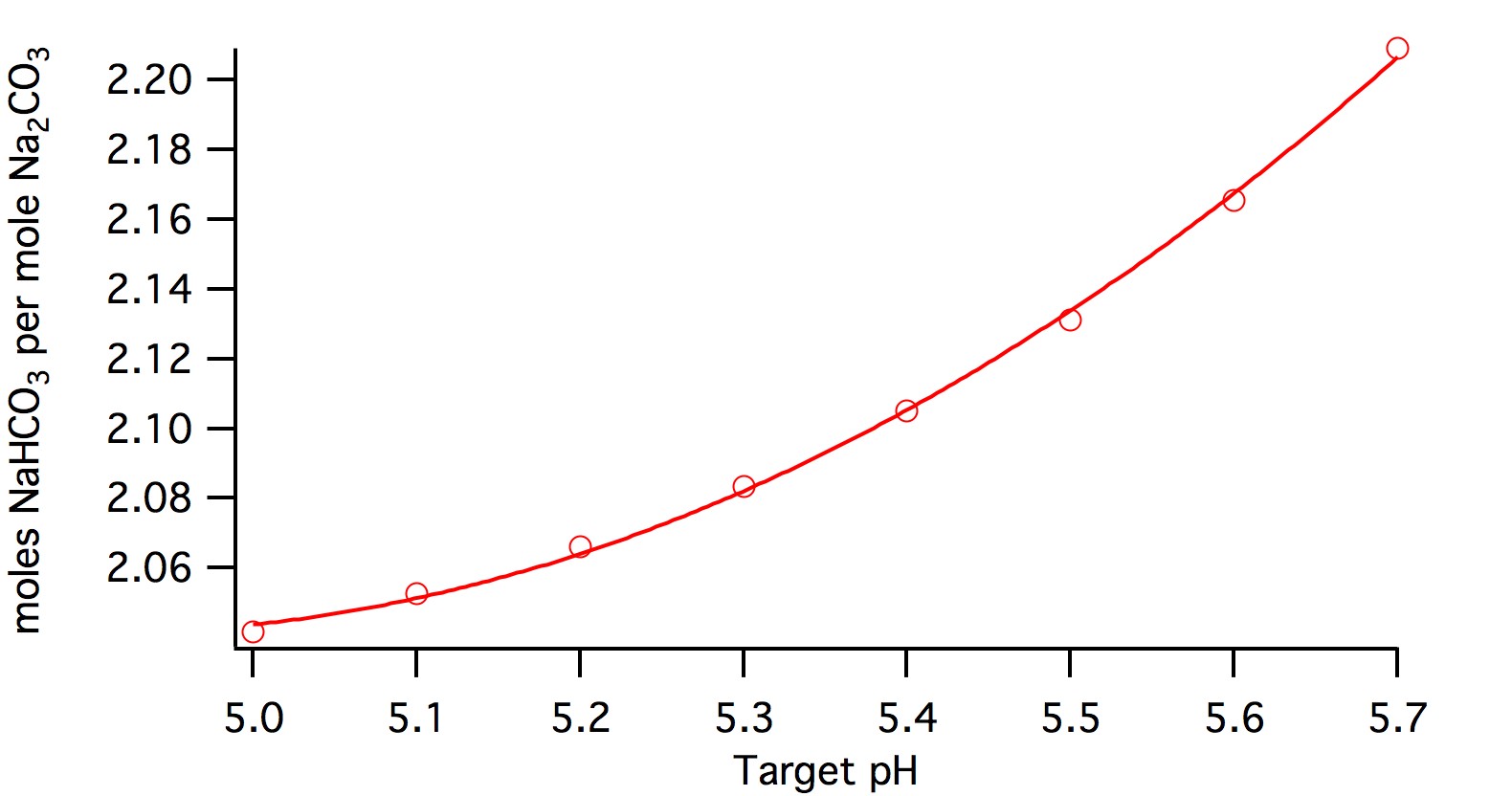
It's more quadratic than linear:
mole_factor = (0.26202*pHz -2.571)*pHz + 8.3482.
Multiply each of those coefficients by the molecular weight ratio (0.84) to get a polynomial for the weight ratio.

It's more quadratic than linear:
mole_factor = (0.26202*pHz -2.571)*pHz + 8.3482.
Multiply each of those coefficients by the molecular weight ratio (0.84) to get a polynomial for the weight ratio.
Yes, with AJ's assistance, the buffering power of baking soda has been properly modeled now in Bru'n Water.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
My next public revision release for Mash Made Easy will have the proper modeling for baking soda as well. As of today my personal use version already has it. Since it is a relatively minor change, with an output impact on the order of ~0.02 pH for a mash target of 5.4, and on the order of ~0.05 pH for a mash target of 5.6, I will wait for some additional changes to accumulate within my personal version before I make this part of a new public release. Likewise, thank you A.J.!
Last edited:
Big Monk
Trappist Please! 🍷
- Joined
- Dec 24, 2015
- Messages
- 2,192
- Reaction score
- 1,154
I have an interesting little brewday sheet coming out that fully integrates AJ’s proton deficit “troubleshooter” into an easy to use brewday sheet including extract, bitterness (boil and whirlpool), color, and full volume accounting.
Stay tuned...
Stay tuned...
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Now that we have resolved the calcium carbonate issue for the OP, I'm questioning whether Brewers Friend can be taken to be correct for this recipe, as in Mash Made Easy version 2.10, and with the presumption of a mash pH target of 5.4, this recipe requires mash water with 2.0 mEq's of alkalinity, provided that I also make the assumption of 0.75 mEq of Ca++ ions being present in the mash water. If the OP modeled this batch in RO water with zero alkalinity I would question such a grist doughing in straight up at a mash pH of 5.37, given the same presumption of 0.75 mEq of calcium present within the mash water.
Lots of past historical data for robust stouts would seem to be more in agreement with bringing the mash water to around 2 mEq of alkalinity for recipes similar to this one.
And I can also remember back when Palmer might have been suggesting something on the order of 250 ppm alkalinity (or higher) mash water for a recipe on the order of this one. That might perhaps be more on the order of 5 mEq's of alkalinity.
Lots of past historical data for robust stouts would seem to be more in agreement with bringing the mash water to around 2 mEq of alkalinity for recipes similar to this one.
And I can also remember back when Palmer might have been suggesting something on the order of 250 ppm alkalinity (or higher) mash water for a recipe on the order of this one. That might perhaps be more on the order of 5 mEq's of alkalinity.
Last edited:
You now have a tool in the form of the troubleshooter/voltmeter that will let you check on the performance of any other spreadsheet. It will show in detail where all the protons go. If you put a simple (1 malt) mash into it with given amounts of acid, baking soda, and calcium and into the suspect spread sheet and don't get the same answer you can then start poking aroung until you find that it isn't handling calcium "correctly" (isn't using the same Kolbach coefficient as the one you put into the trouble shooter) or that it isn't handling bicarb correctly, that the malt you have chosen is sufficiently non linear that the linearized solution introduces appreciable discrepany etc. You will probably come down to the conclusion that the difference is in the malt model and as we have noted here before no fix to a spreadsheet can repair poor malt modeling. Of course fixing a spreadsheet's ability to more accurately model malt titration curves will improve its performance but only if it is given good data.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
I just entered the following into Kaiser Water Calculator version 1.58 to simulate the OP's recipe:
26 lbs grist
80 SRM batch final color
8.5 gal mash water, DI
3 gal sparge water, DI
3.25 g. Gypsum added to mash only
3.25 g. CaCl2 added to mash only
90% of batches SRM color contribution assigned to coming from exclusively the grists roasted malts/grains
(after properly excluding any SRM color which might stem from base malts, as per the explicit instructions seen on the Kaiser calculator, and including only crystal and roasted SRM contributions, also per the instructions)
The output from the Kaiser Calculator for this input is as follows:
-------------------------------------------------------------------------------------
Pre-adjustment mash pH = 5.06
16.5 g. baking soda addition required to mash at pH 5.4, or roughly 5.8 mEq
Note: If less than 90% of SRM is assigned from roasted, the baking soda needed to hit 5.4 pH in the mash goes up, and is greater than 16.5 g.
26 lbs grist
80 SRM batch final color
8.5 gal mash water, DI
3 gal sparge water, DI
3.25 g. Gypsum added to mash only
3.25 g. CaCl2 added to mash only
90% of batches SRM color contribution assigned to coming from exclusively the grists roasted malts/grains
(after properly excluding any SRM color which might stem from base malts, as per the explicit instructions seen on the Kaiser calculator, and including only crystal and roasted SRM contributions, also per the instructions)
The output from the Kaiser Calculator for this input is as follows:
-------------------------------------------------------------------------------------
Pre-adjustment mash pH = 5.06
16.5 g. baking soda addition required to mash at pH 5.4, or roughly 5.8 mEq
Note: If less than 90% of SRM is assigned from roasted, the baking soda needed to hit 5.4 pH in the mash goes up, and is greater than 16.5 g.
Last edited:
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
It would be helpful if the OP gave us the gallons of mash water, the expected finished batch size, and the anticipated SRM color for the finished beer. I could then re-enter the corrected data into the Kaiser calculator to correct for these input values vs. my presumptions.
Big Monk
Trappist Please! 🍷
- Joined
- Dec 24, 2015
- Messages
- 2,192
- Reaction score
- 1,154
It would be helpful if the OP gave us the gallons of mash water, the expected finished batch size, and the anticipated SRM color for the finished beer. I could then re-enter the corrected data into the Kaiser calculator to correct for these input values vs. my presumptions.
After our discussion and development of A.J.’s troubleshooter, why even bother with Kai’s sheet?
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
RPIScotty, would you be able to input the OP's recipe into your version of the troubleshooter, to see how many mEq's of alkalinity it indicates, if any, to hit 5.4 pH on the nose within the mash?
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
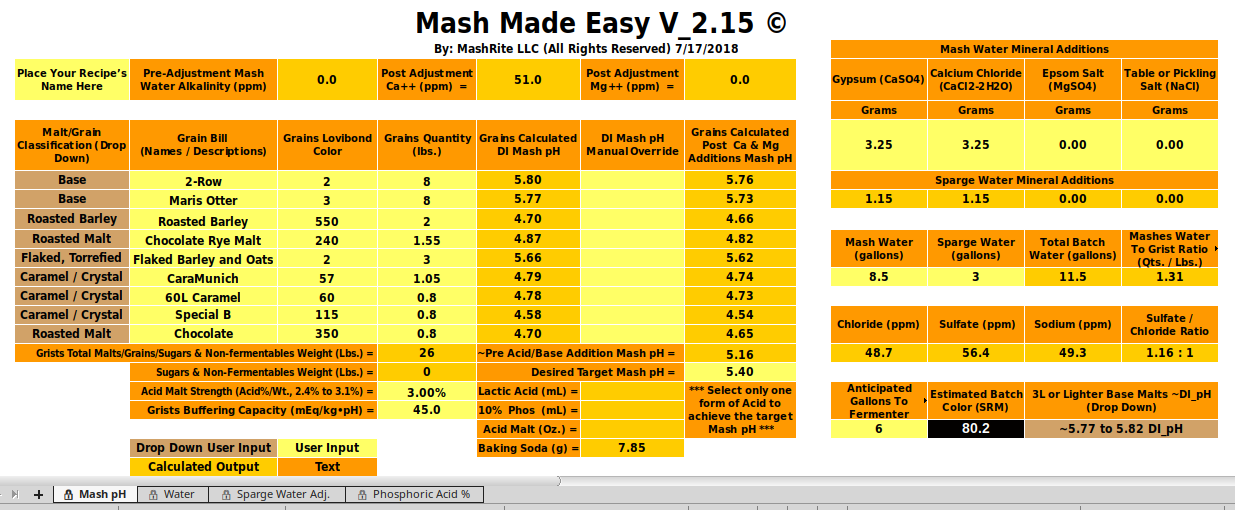
I just plugged the OP's recipe into Mash Made Easy, and its output can be seen below. It's calling for half the baking soda that the Kaiser Calculator calls for. Note, this is a testing version of MME, and not a public release. The only difference between this version and the latest released version is the application of A.J.'s baking soda absorbance factor formula, which bumps up the baking soda grams a bit.

Big Monk
Trappist Please! 🍷
- Joined
- Dec 24, 2015
- Messages
- 2,192
- Reaction score
- 1,154
I just plugged the OP's recipe into Mash Made Easy, and its output can be seen below. It's calling for half the baking soda that the Kaiser Calculator calls for. Note, this is a testing version of MME, and not a public release. The only difference between this version and the latest released version is the application of A.J.'s baking soda absorbance factor formula, which bumps up the baking soda grams a bit.
View attachment 579736
EDIT: Error.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
While the immediately above posting error is being resolved, let me correct a minor error of my own.
When I manually set 2-Row to a DI_pH of 5.63 and Maris Otter to a DI_pH of 5.75 in 'MME' the baking soda required to move the mash to a pH of 5.4 jumps up a bit, and becomes 8.44 g.. These are likely a bit more realistic choices for DI_pH for these two specific base malts than those seen in my initial snapshot above.
Essentially the very same output can be accomplished by selecting a base malt DI_pH range of "5.68 to 5.72" (splitting the difference as to this selection) via to the selector drop-down in the lower right corner, and performing no manual overrides. For this case the output advice is to add 8.41 g. of baking soda. Splitting hairs vs. the above.
These slightly increased baking soda requirement values are to be preferred to my more rapidly developed initial presentation of MME for this recipe, as in my first attempt (see snapshot above) I did not properly pay attention to the base malt selector, and it wasn't set for the best choice with respect to the two base malts in this recipe.
I currently tend to brew using Swaen Pilsner malt as my base for everything, because I have a load of it on hand, and at present it is my only base malt that I have in house, and it works well with the higher selector setting I had originally set, so that's how MME mistakenly got set to the "5.77 to 5.82" base malt DI_pH range as seen in the above snapshot.
When I manually set 2-Row to a DI_pH of 5.63 and Maris Otter to a DI_pH of 5.75 in 'MME' the baking soda required to move the mash to a pH of 5.4 jumps up a bit, and becomes 8.44 g.. These are likely a bit more realistic choices for DI_pH for these two specific base malts than those seen in my initial snapshot above.
Essentially the very same output can be accomplished by selecting a base malt DI_pH range of "5.68 to 5.72" (splitting the difference as to this selection) via to the selector drop-down in the lower right corner, and performing no manual overrides. For this case the output advice is to add 8.41 g. of baking soda. Splitting hairs vs. the above.
These slightly increased baking soda requirement values are to be preferred to my more rapidly developed initial presentation of MME for this recipe, as in my first attempt (see snapshot above) I did not properly pay attention to the base malt selector, and it wasn't set for the best choice with respect to the two base malts in this recipe.
I currently tend to brew using Swaen Pilsner malt as my base for everything, because I have a load of it on hand, and at present it is my only base malt that I have in house, and it works well with the higher selector setting I had originally set, so that's how MME mistakenly got set to the "5.77 to 5.82" base malt DI_pH range as seen in the above snapshot.
Last edited:
Similar threads
- Replies
- 3
- Views
- 733
- Replies
- 3
- Views
- 1K
- Replies
- 10
- Views
- 937
- Replies
- 52
- Views
- 5K