Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
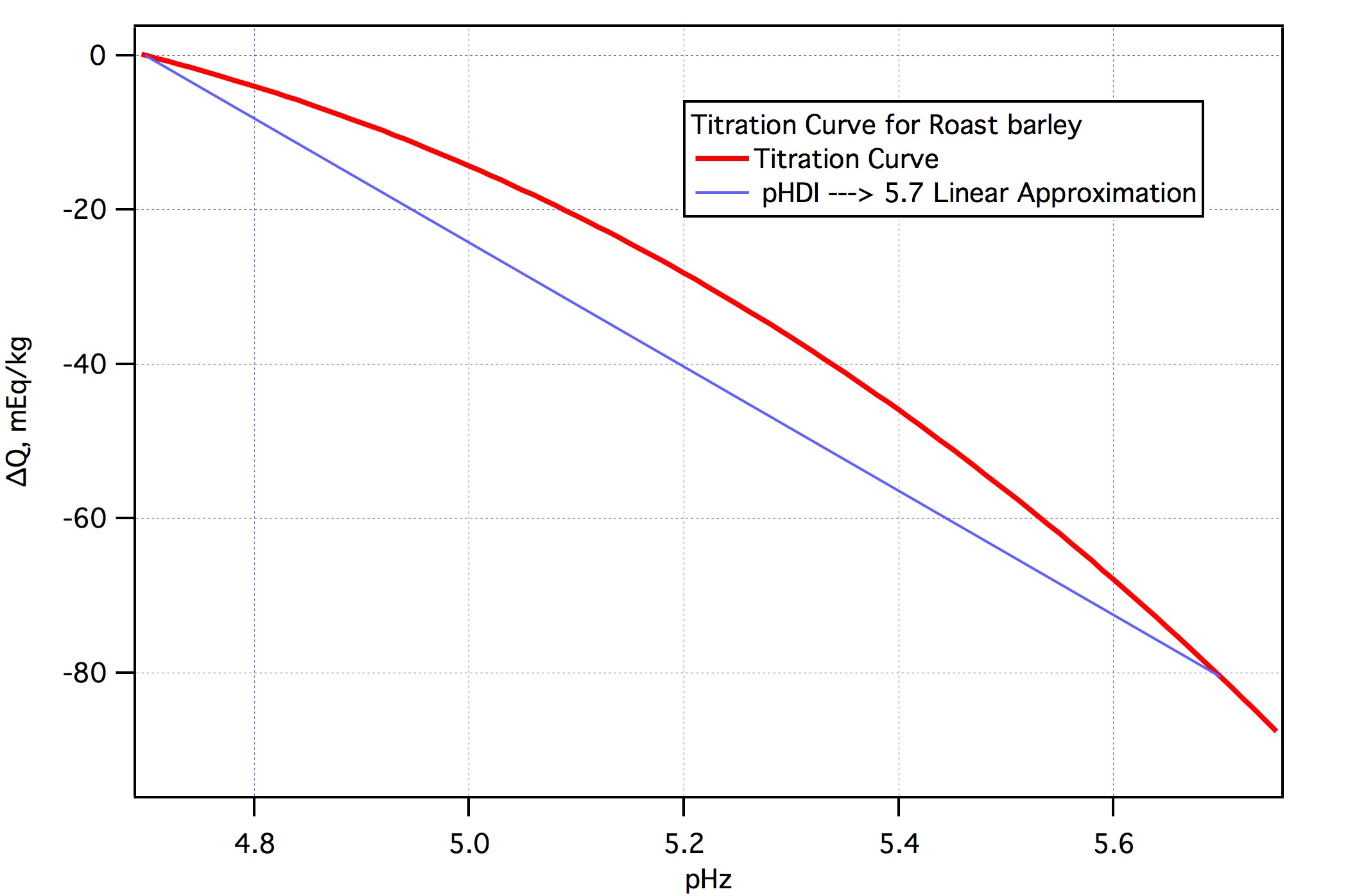
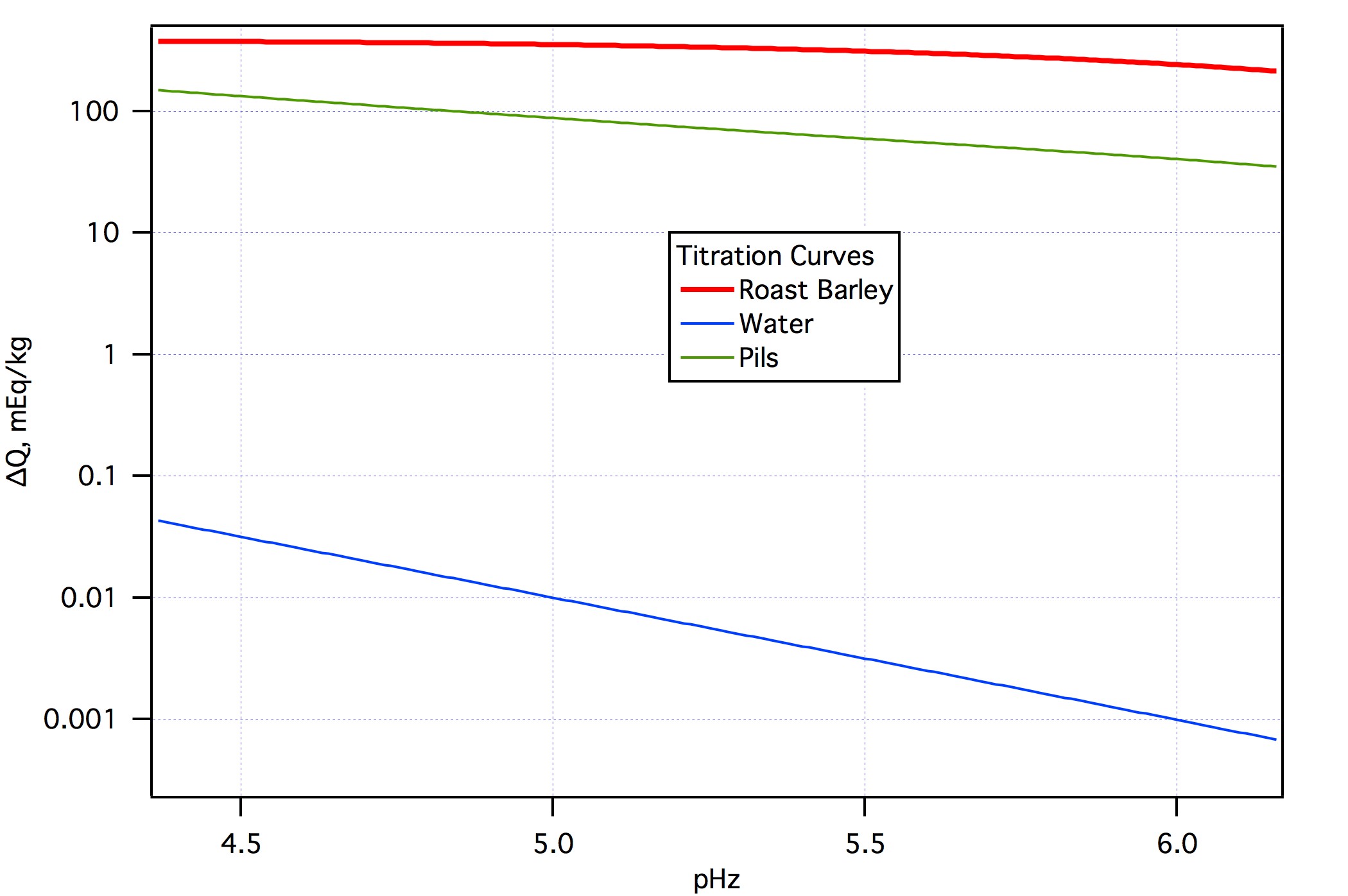
If Brewers Friend is used as in the very first of the OP's posts to this thread, but instead is set to the quick and easy "SRM color" mode, it gives effectively identical output as does the Kaiser Water Calculator, with my result for this Brewers Friend effort being the need for the addition of 16 grams of baking soda to hit a mash pH of 5.4.
This is not surprising, as Kai Troester, the very same Kai who created the Kaiser Water Calculator, contributed this section of the Brewers Friend online calculator.
But this leads to the conundrum of one BF method advising (perhaps, as I'm merely guessing here) 0.5 g. of baking soda to bump it from 5.37 to 5.4 pH, and another BF method offered within the same calculator advising the addition of 16 g. of backing soda, to bump it from 5.05 pH to 5.4 pH.
This is not surprising, as Kai Troester, the very same Kai who created the Kaiser Water Calculator, contributed this section of the Brewers Friend online calculator.
But this leads to the conundrum of one BF method advising (perhaps, as I'm merely guessing here) 0.5 g. of baking soda to bump it from 5.37 to 5.4 pH, and another BF method offered within the same calculator advising the addition of 16 g. of backing soda, to bump it from 5.05 pH to 5.4 pH.
Last edited:


![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)