sarandos25
Member
- Joined
- Feb 6, 2021
- Messages
- 21
- Reaction score
- 7
Yes, I didBTW you did crush the grain, right?

Yes, I didBTW you did crush the grain, right?
I think @Peebee is onto something re: low Alkalinity. In the 10s of hours I've spent reading blogs and materials on waters I believe I read that this might result in low pH. The only lab test I have conducted did tell me that my Total alkalinity was about 8 mg CaCO3/L - could this be the driver?I'm no expert, but ISTM that your water report has to be wrong.
Nice job. The mystery deepens.I think @Peebee is onto something re: low Alkalinity. In the 10s of hours I've spent reading blogs and materials on waters I believe I read that this might result in low pH. The only lab test I have conducted did tell me that my Total alkalinity was about 8 mg CaCO3/L - could this be the driver?
I sent a new water sample today to a lab to get all the stats, but that will take 2 weeks to come back...
Even then, I wonder why the slaked lime has made no impact, although I wasn't aware about how CO2 can impact it so it may be degraded and that's why it doesn't do anything...
Nice job. The mystery deepens.
Even if you mashed with distilled water it should not be that low.
I'm not sure what could be in your tap water that would cause it to be that low. Massive amounts of calcium? You're getting it tested again, but maybe you could do a test mash with distilled water if available? I should be able to do this test, what pH should I expect?
We've ruled out your other water additives now.
So that leaves grain -maybe a weird batch that's soured somehow? No. I use this pale ale for a WIPA, NEIPA, Cream Ale, all 3 suffer the same issue, that's about 20 batches, so unless Weyermann in general is prone to be acidic, the issue is not isolated to my current batch of pale ale.
Calibration solutions - maybe one is off/bad? What are the odds, they are new, bought today for the experiment
pH meter - somehow defective reading in between the calibration points? Or anything about the model that would mean it isn't good for measuring wort? Maybe, Milwaukee 102 will take a month or so to get to Colombia
Other equipment - sample jar has some residue in it? All 4 samples were in different receptacles, I would say the odds would be very low.
What else could we be missing and how can we test it?
No, all 4 samples were measured at 25C.Thank you
Very comprehensive.
Assuming the sample was cooled to 20C before pH reading. Edit I see it was cooled sufficiently.
You rinsed the pH meter off before using it and between tests?
If the reading hasn't been changed by temp or no rinse then it's either your water or the grains.
What's the pH of the water with the calibrated meter?
Have you tasted that pale malt ? Could it have started to sour or been contaminated with sour malt?
If it has it will taste " acidy" .










I haven't made myself clear. Those batches have been across several months, and I have purchased malt multiple times. Some batches in late 2023 were made with Pale Ale with a "Datum Produktion: 10.Jan.2023"; the stuff I have right now was bought in Q4'24 has a Produktion in 2024... i.e. the issue spans multiple harvests...@sarandos25
The fact that you say pH issue has been occurring for all of your batches whatever style could mean that a whole sack has a problem.
Taste it, sour malt tastes acidic.
I'm not sure what could be in your tap water that would cause it to be that low. Massive amounts of calcium? You're getting it tested again, but maybe you could do a test mash with distilled water if available? I should be able to do this test, what pH should I expect?
We've ruled out your other water additives now.
So that leaves grain -maybe a weird batch that's soured somehow? No. I use this pale ale for a WIPA, NEIPA, Cream Ale, all 3 suffer the same issue, that's about 20 batches, so unless Weyermann in general is prone to be acidic, the issue is not isolated to my current batch of pale ale.
Calibration solutions - maybe one is off/bad? What are the odds, they are new, bought today for the experiment
pH meter - somehow defective reading in between the calibration points? Or anything about the model that would mean it isn't good for measuring wort? Maybe, Milwaukee 102 will take a month or so to get to Colombia
Other equipment - sample jar has some residue in it? All 4 samples were in different receptacles, I would say the odds would be very low.

I had better preen my best feathers for a bit of strutting about ...I think @Peebee is onto something re: low Alkalinity.
Not really. But I'd agree the "mystery" perhaps needs digging out of its shallow grave?The mystery deepens. ...
I had better preen my best feathers for a bit of strutting about ...
Not really. But I'd agree the "mystery" perhaps needs digging out of its shallow grave?
Okay, enough talking in riddles, and trying to take credit for coming up with a "novel" idea. All I've done is resurrected some dusty features in "Bru'n Water" ... "Add Sparging Mineral Additions to the Mash" and, more importantly, "Add Hardness Minerals to the Kettle"... There were reasons for these features, but they appear to have been forgotten because I had to come up with my own reasons to use them (I couldn't find any guidance).
Lots of speculation about the causes of @sarandos25's problem: pH? Unusual grain? But pH has a somewhat tentative connection with what's being done here (the reason I've just got myself a load of less accurate brewing pH indicator papers to use instead of my meter). Different "calculators" are going to arrive at different pH predictions, and, rather handily, the reasons are grain! Remember who is the "Original Poster" (or Original Post's author ... I can never figure out what "OP" stands for?), @Silver_Is_Money (I was only chatting to him a few weeks ago, so he knows why I'm going off on one here!). Here's a thread he's in, relevant to what I've just said: https://www.homebrewtalk.com/thread...iffe-and-spencer-in-2018.702832/#post-9318458
("Quasi-empirical" ... one of his favorite words? One of mine too now I've Googled what "quasi" means ... it's what I do! "Apparently but not really").
So, I don't believe these pH, grain, etc. explanations here, I reckon it's to do with @sarandos25's use of highly mineralised brewing water (Calcium at 120ppm ... more like I'd do being a Brit brewing "traditional" British style beer). All that Calcium and tap water alkalinity at a low starting point (the latter also possibly affecting how the brewer may react), that's where I see the problem lies (Calcium + Malt grains = pH going down!) .
And of course ... I could be wrong!
The last comment is because this topic is dangerously close to the topic that has recently got me thrown off the UK "sister" forum! The UK "THBF". And it was me reporting the infringement of forum rules! So, I'm being careful here, though I do trust the moderators here to handle things "differently".
OK... really excited to share the results; curious to see the reactions to them:
Test:
500gr Weyermann Pale Ale
1.5 liters of source tap water
0 salt additions
pH Calibration
View attachment 866412
View attachment 866413
Additional visual validation:
Pale ale
View attachment 866414
Mashed at 67 C for 60 Minutes
View attachment 866416
Now the interesting things:
Mash pH 20' measured at 25 C
View attachment 866418
Mash pH 30' measured at 25 C
View attachment 866419
Mash pH 45' measured at 25 C
View attachment 866420
Mash pH 60' measured at 25 C
View attachment 866421
Based on the calc the pH should be clocking in at 5.7...
View attachment 866422
View attachment 866423
Help please!!

Best answered by @sarandos25, but I assumed the "brewing water" (source water) was low (2ppm), my reply was referring to the target profile (120ppm). And ... ahhh!If the water testing was correct there is low Ca in the brewing water.
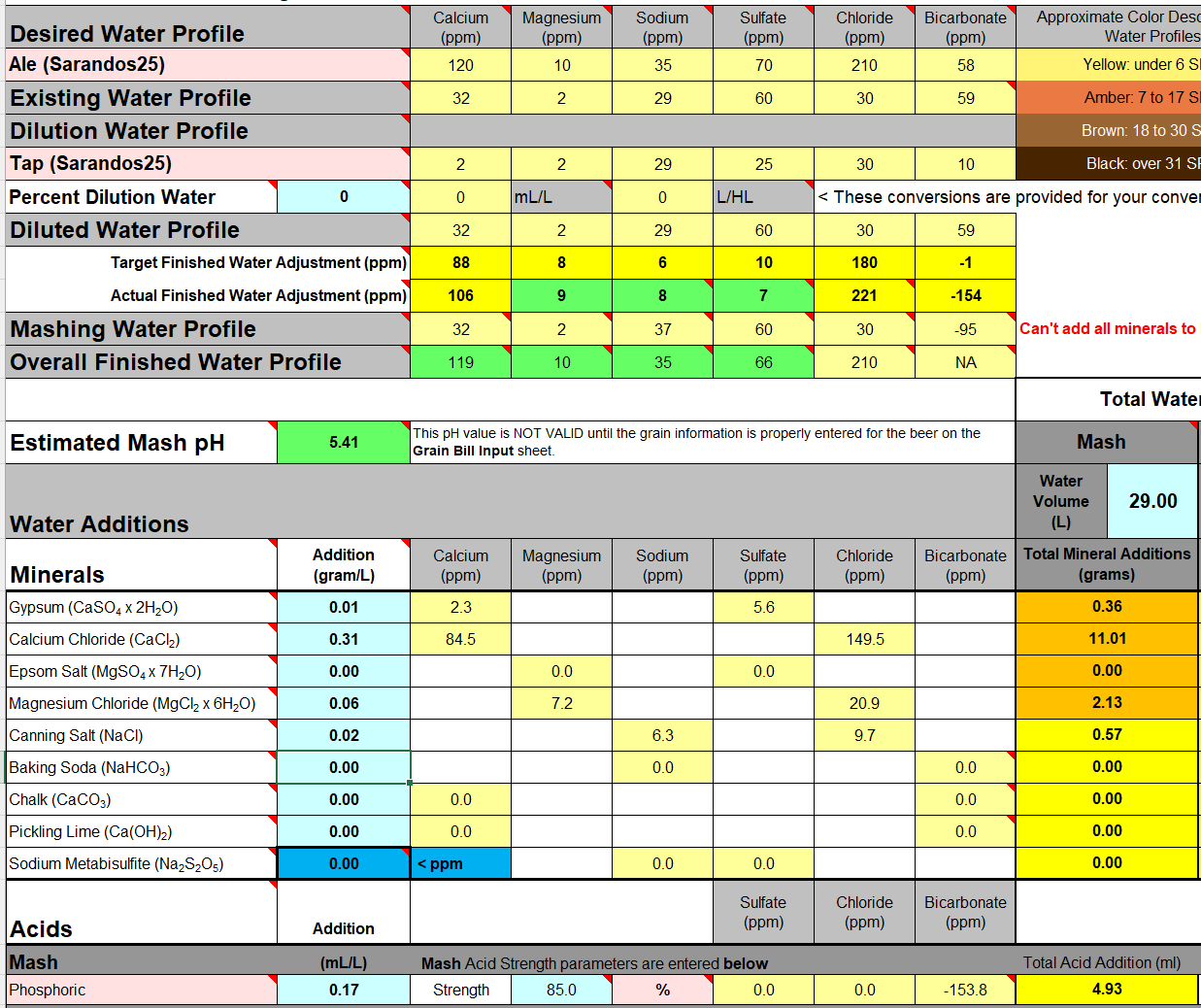
Thanks. But I'm already using Alkalinity as 8ppm as CaCO3, must have picked it up at some point.
I'm still working with the full-size batch @sarandos25's original post laid out, not the mini-mash stuff.@Peebee
I think the point is the base water is pretty bland and used in a mini mash with NO salts added to the tap water the measured pH is still lower than predicted.
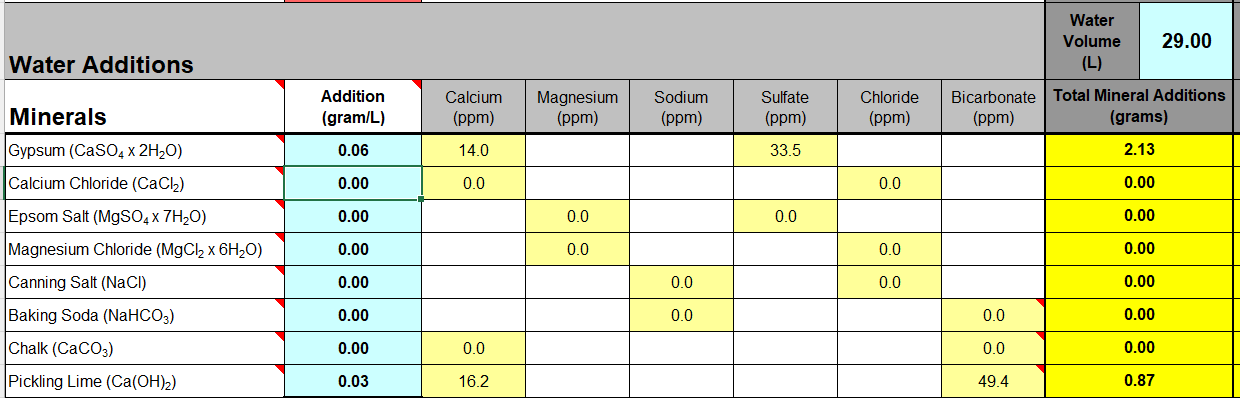
My money is on a measurement generated wrong value. The new pH meter and retest should clarify.I catch up eventually!
Mini-mash: Yeap, confirm pH is calculated in Bru'n Water as 5.70. Actually appears to be 5.02 in practice. That is nothing to do with what I'm messing about with, it would require (theoretically - if there's enough "stuff" in the malt to react with) Calcium ions to go up by about 298mg/l to achieve that (according to Bru'n Water). Seems very (very³?) unlikely.
Erm ... well, I've nothing more to add?
I wondered that last night after posting (and re-reading Cire's posts); it's a long time (years) since those "pen testers" required such care calibrating. Now-a-days the testers are just dropped into a buffer solution, it recognised the buffer, and calibrates accordingly? The probes on those pens have a finite life (about 6-18monthes) whether you use them or not. So, are any of those old-style 3-point calibrated pen-testers still in-date?My money is on a measurement generated wrong value. The new pH meter and retest should clarify.
He's got a Milwaukee on order.I wondered that last night after posting (and re-reading Cire's posts); it's a long time (years) since those "pen testers" required such care calibrating. Now-a-days the testers are just dropped into a buffer solution, it recognised the buffer, and calibrates accordingly? The probes on those pens have a finite life (about 6-18monthes) whether you use them or not. So, are any of those old-style 3-point calibrated pen-testers still in-date?
If it's over a year old, whether or not it holds calibration longer than a few minutes, bin it! A "new pH tester" is in order!