Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
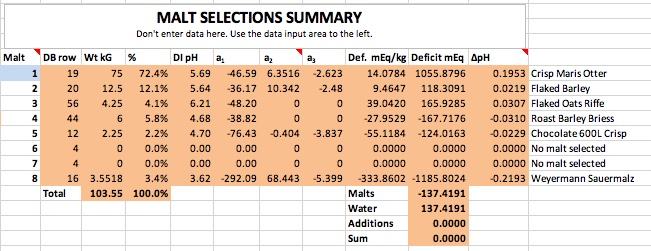
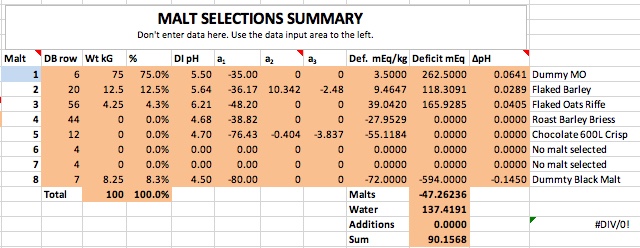
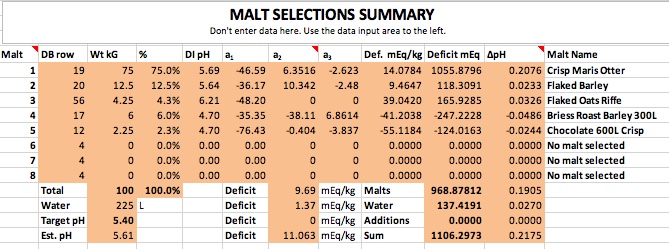
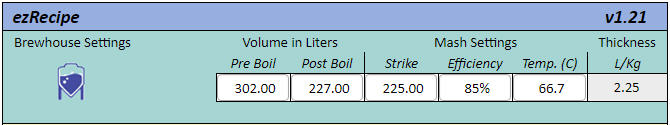
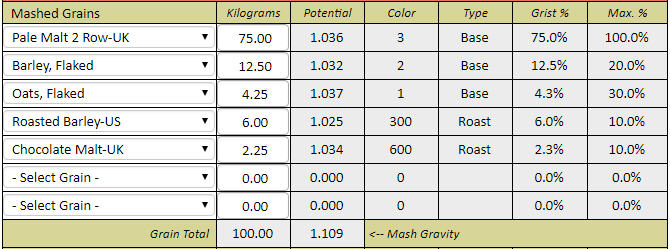
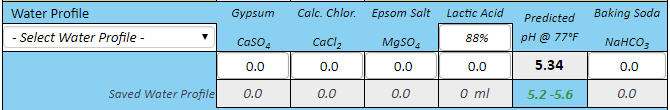
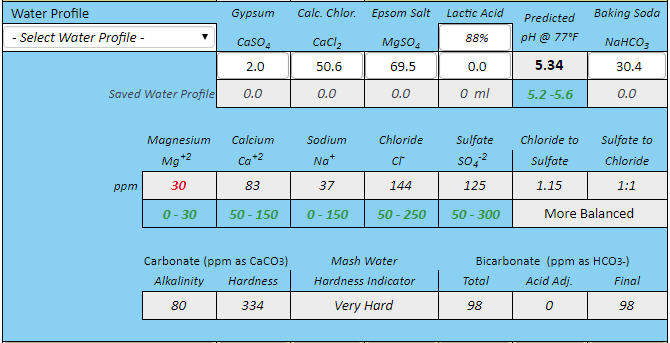
If you want to synthesize water with 100 ppm alkalinity for some reason then do it shooting for 8.38 pH. Then you only need 2.53 grams of bicarbonate and no acid at all.
Thanks for correcting me. Then the correct answers are 2.53 grams of baking soda for 4 gallons, and 5.06 grams for 8 gallons to create 100 ppm alkalinity water at these respective volumes. The essence of this is that to hit pH 5.4 with his stout recipe forum member 'cire' was in effect starting with water that had this much baking soda (or actually its equivalent) already added to it by nature and locational circumstance. That is the real point I was trying to make here. I had no intent to suggest that acid was required, sans that brought to the mash by the grist, but rather to emphasize that if this level of bicarb is required for the posters recipe to hit 5.4 pH in the mash, it would not have doughed in at 5.4 pH given a mash in RO or distilled water, but rather at something rather noticeably lower than this. Perhaps even at 5.0 pH or lower.
But then what has become of your baking soda modifier formula in this case? Would not it require (per your formula) about ballpark a bit more than 5% more than 2.53 or 5.06 grams of baking soda respectively (for 4 gallons of mash water, and for 8 gallons of mash water) for cire to hit 5.4 pH during his mash, if he had started with RO or distilled water instead of his naturally sourced 100 ppm alkalinity water? This is how I initially derived 2.68 grams and 5.36 grams respectfully of 4 gallons and 8 gallons.
I'm expressing the independent cases for 4 gallons and 8 gallons only because I do not know if cire sparges or does no-sparge, so I guessed at 4 gallons of 100 ppm alkalinity mash water for the case of sparging, to be followed by 4 gallons of sparge water, and I guessed separately at 8 gallons of 100 ppm alkalinity water for the case of no sparge, and for both cases with his naturally 100 ppm alkalinity sourced water, but while looking at duplicating his 5.4 mash pH from the perspective of starting out with RO or distilled instead.
I hope the above brings clarity to what I had written earlier that confused you.
Last edited:























































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)