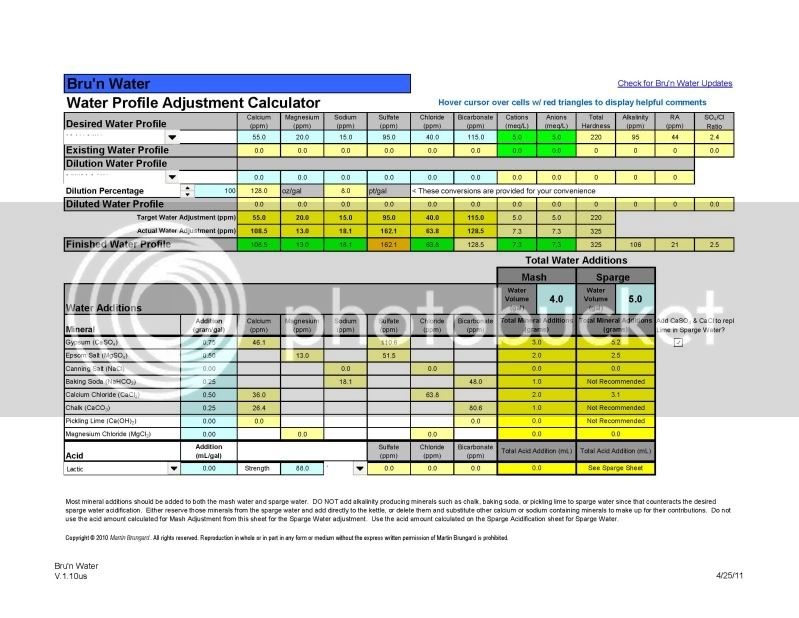
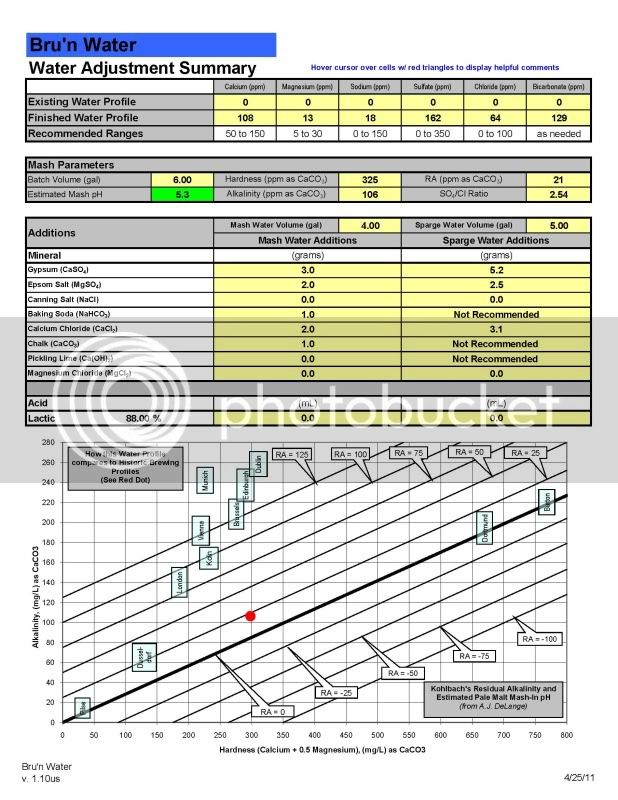
Salt additions for ppm in your water is simple and easy (I mean its just inorganic aqueous chemistry). But how the interaction with the malt and the water profile effects mash pH is more complicated, depending on the malt itself, which is organic.
If you are interested in hitting pH values you must have a good pH meter (and take readings at the reference temp.). Only then can you tune in your system with your malts and your water. So, using sauermalt or lactic acid without a pH meter is shooting in the dark. Doesn't mean its going to make bad beer, but it doesn't mean its going to make good beer either.
If you are solely interested in salt additions to make hops pop or bring the malt forward stick with the spreadsheets. But know that salt additions have the potential to effect your mash pH, which can effect the quality of your beer in the end. If you think the salts may be messing with your pH, get a pH meter and use it every time you brew.
If you have soft water: dark beers (a lot of roasted malts) will be an issue in terms of pH being too low.
If you have high alkalinity water: light beers will tend to be a problem (too high pH)
***The last two lines are way over simplified***
Well, my light beers all tend to carry an unpleasant thinness to the mouthfeel and flavor, even when fully carbed. This is why I'm trying to grok water chemistry so I can know if I'm doing the right thing or not.
The OP seems to say distilled + CaCl2 for very light beer, and that's going to get you very good beer, and don't sweat it. And I get people telling me spreadsheets are no good if you don't know the science.
But then I get a couple people on here telling me the more precise salt additions and the spreadsheets are good but you don't need to be exact, and you do need to get a pH meter to get the proper wort acidity.
On the specific water chemistry thread for my brew next Saturday, I worked out a series of salt additions using the Bru'n Water sheet. Then I get people jumping on to tell me I'm overthinking it and to forget about everything except for the CaCl2 and sauermalz as predicted by BeerSmith. Then on this thread I get people telling me, variously, that the spreadsheet stuff isn't stuff I need to worry about, and it's flawed anyway, or that some spreadsheets are more or less flawed than others, and that it IS stuff I need to worry about, but not to the point of obsession.
And regarding pH, some of these spreadsheets and calculators will let you put in your grain bill and give you the resulting mash pH from the treated water, and tell you how much sauermalz to use to get into the ideal range. So those of you telling me a pH meter is absolutely vital to getting the right wort acidity, is it just because the spreadsheets aren't accurate enough to trust?
So yeah, my head is spinning in circles and I'm going to break for lunch.
And for the record, I'm going to take the distilled + CaCl2 + sauermalz approach this weekend unless someone tells me it's a bad idea.