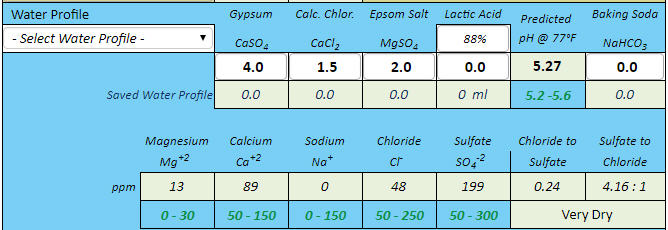
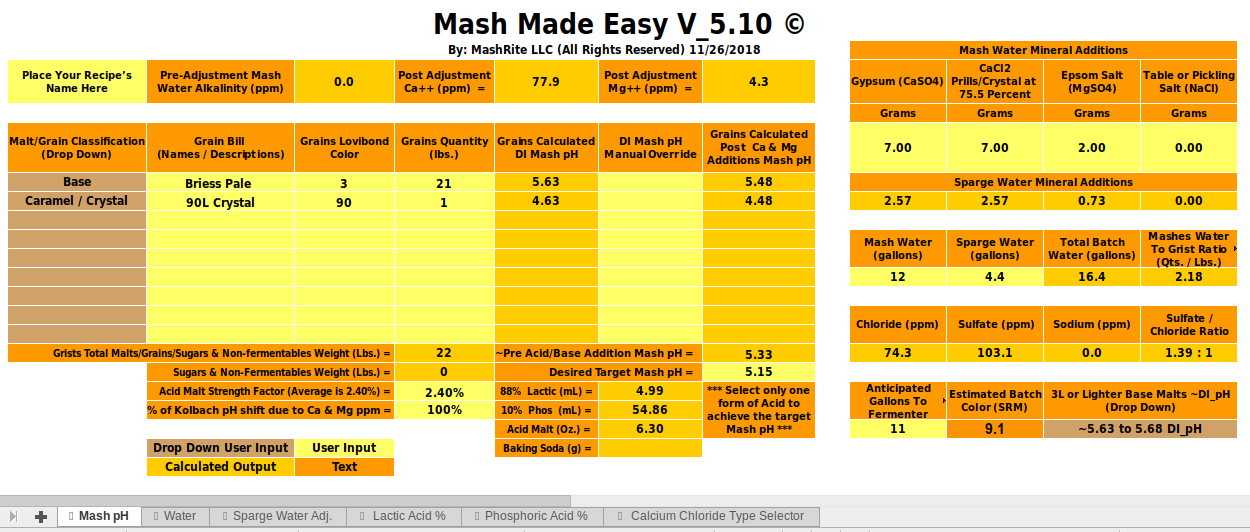
I'd imagine this is probably a common issue, but I entered my next brew into several different water chemistry calculators (EZ Water, Brun Water, MpH, brewer's friend) and seem to be getting quite the range of predicted pH's.
It is indeed the case.
EZ Water: 5.45
BrunWater: 5.07
MpH: 5.40
Brewer's friend: 5.46
Obviously, BrunWater seems to be the gross outlier. Does anyone know why this may be the case?
Yes. It has been discussed here extensively and in the Brewing Softrware forum as well. In order to predict mash pH reliably you need two things:
1. A program which bases its calculations on the chemistry of the mash
2. Data which accurately describes the malts you intend to use.
The programs you tried have neither. Of them, only the author of Brewer's Friend really seems to understand the chemistry. The others appear to use quasi empirical approaches to modeling mash chemistry which include a number of approximations. One common to all of them, including Brewer's friend, is that malts' titration curves are linear. They are, approximately, linear and in some cases considering them to be so introduces only minor errors but in other cases the errors so induced are larger. Other approximations are linear relationships between water's alkalinity and its bicarbonate content, the assumption that acids and bases have the same strength regardless of pH. Thus the calculators themselves are not robust.
Reliable malt data can only be obtained by measuring the malts. A few people have done some of that but they have not measured the bag you are using so that their measurements can only typify malt characteristics but cannot accurately model the malts you will actually use. Measuring malt characteristics takes a fair amount of work an based on my experiences here trying to coach home brewers through the task is very difficult because they don't understand the chemistry well enough to be able to follow the instructions other than blindly. That aside, it is much less trouble to make a test mash and measure its pH than it is to measure all the malts adequately and insert them into a program that can accept this data. There is no such program currently available.
Most of the available programs try to model the malts' acid/base properties in terms of their color (Lovibond rating) or type (base malt...). While there is correlation between color and DIpH (one of the important malt parameters). It is not nearly tight enough to use as a predictor of DI pH.
Am I entering the inputs incorrectly into BrunWater?
That's always a possibility that you should consider. This particular program has a unique and eccentric way of treating water alkalinity so be sure you are handling that in the way the program wants.
Why else would it be this way off?
No one really knows how this program works and the author isn't talking. From his frequent contributions to this forum we can sort of deduce that its algorithm is based mostly on empirical methods based on the experiences of people who use his program. Earlier this year people here reported that they were finding that increasing the DI water to grist ratio caused it to predict lower pH. This clearly cannot happen. The author reported that fixed. Another reader here found with it that equivalent amounts of two different basic salts caused it to estimate different pH shifts. Clearly this is wrong. Several people have, as you did, reported pH predictions, especially with dark malts, that are much lower than makes sense. Author's response to this is that he needs to get more reports on dark beer mash pH from his users in order to be able to fix this. All of these solidify the impression that the algorithm is empirical rather than based on the chemistry. The general problem with empirical solutions is that they tend to be valid only under certain conditions - those under which the data underlying them were gathered. When those conditions aren't met, the creator often tries to patch the algorithm to "fix" the problem by "tweaking" it to incorporate the outliers. Often this causes another problem to pop up. I think that's what happening here.
I just started brewing, so I don't have a pH meter to measure pH.
If you take anything away from all this I hope it will be that a pH measurement on a test mash is going to be much more helpful to you in planning your brews than any calculator or spreadsheet.
As you may have gathered I am not very sanguine towards the liklihood of a really useful brewing calculator any time soon. The fundamental problem is that even were a robust calculator prepared there would be no good malt data to put into it. The only way we could ever hope to get good malt data would be if the maltsters would measure it and include it in their malt spec sheets. They aren't about to do that. Even of they did, to make a spreadsheet that uses that data requires understanding of the chemistry. I don't quite know why but this chemistry is very difficult to understand.