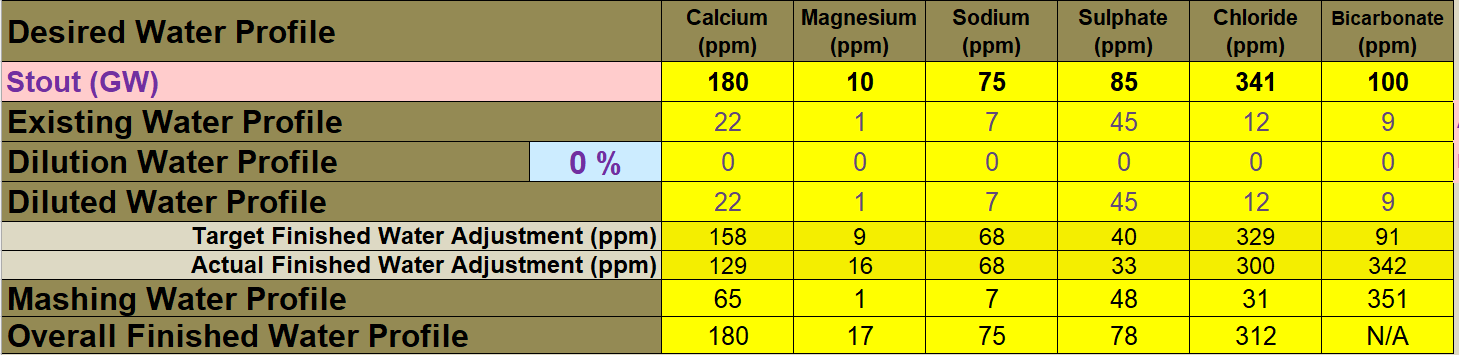
The original thread (seven-month-old) doesn't need waking up, but to continue where it left off ...
I've been hacking away at the subject for a while now; I've not given up! It was a rant about home-brewer's careless mixing of "concentration" units with explicit units and the chaos that's resulting (like people measuring salts to accuracies of a hundredth of a gram ... and thinking it's doing any good!). It's taken me a while to collect enough to offer something more reasonable ... and that's because the errors are so deep ingrained, I couldn't help but get confused myself. I had a reason to keep hacking at it, I was getting "impossibly" low mash pHs (below 5.0).
For assistance I was (mis)using a respected water calculator ... Bru'n Water. As I do not have permission from Martin Brungard yet (?) I've to stick to pictures. The "parasitic spreadsheet" I created is perhaps "too close to the line" (not just the look and feel) to duplicate publicly?
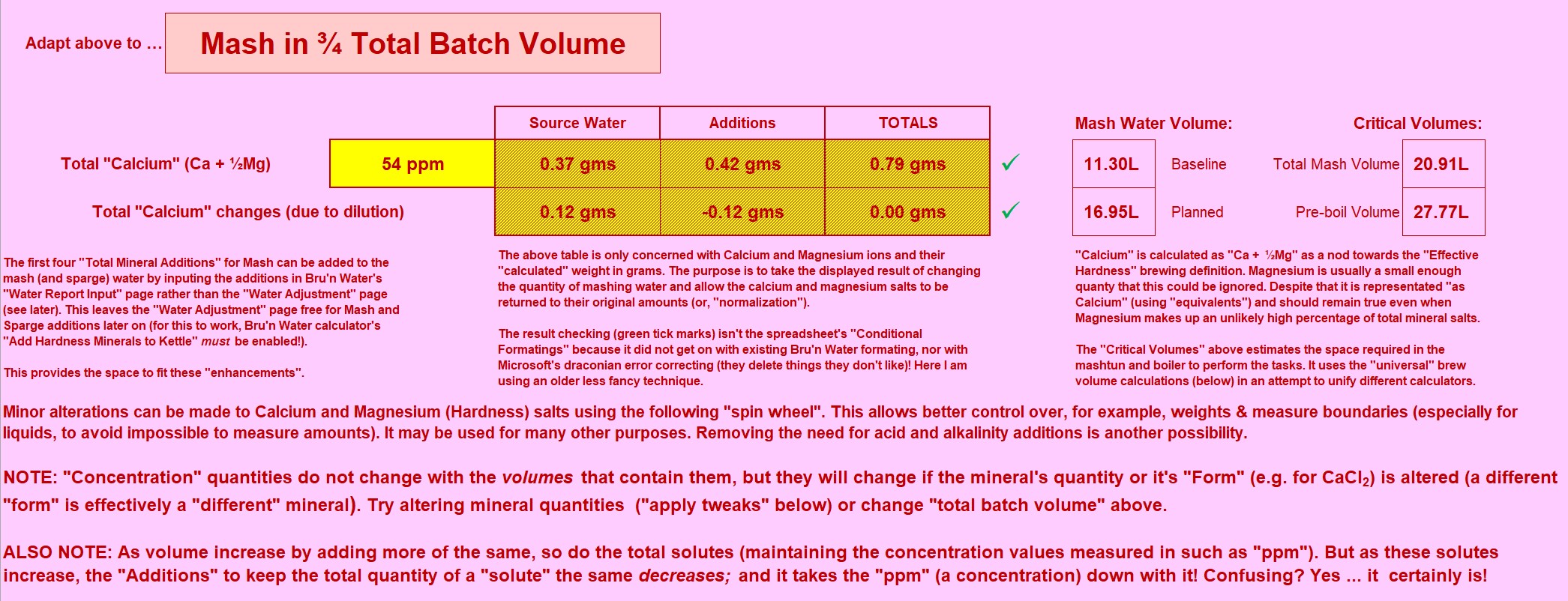
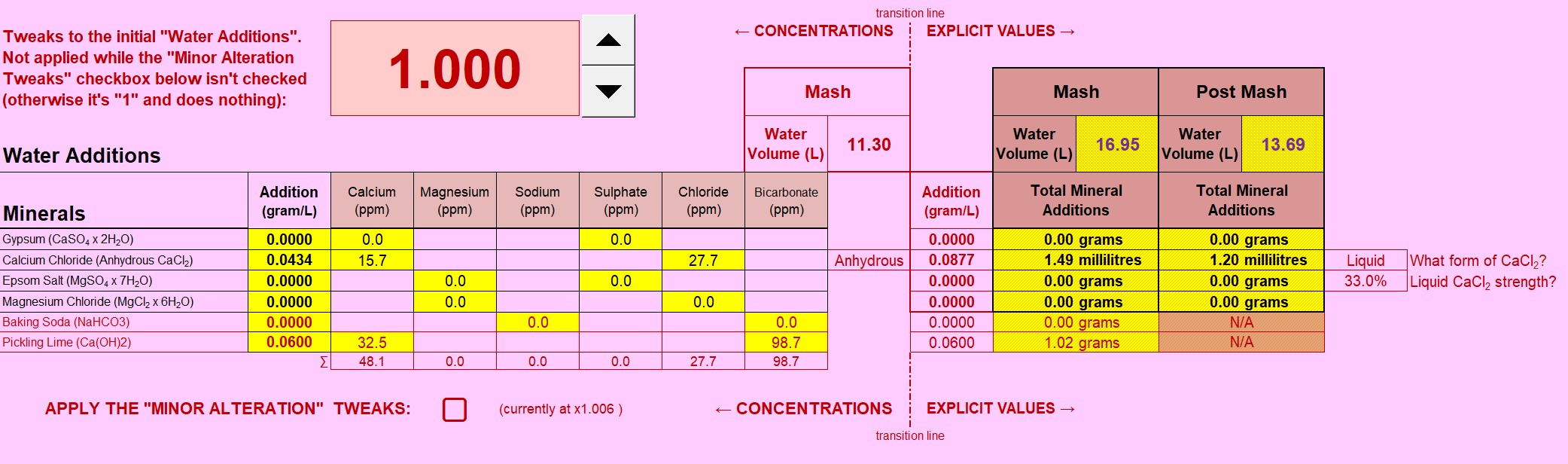
The first thing I attempt to do is create a "baseline". I need the baseline to calculate salt additions without using "concentration" units. Anyone who attempts to measure salts to "hundredths of a gram", "tenths of a gram" or even less "accuracy" for that matter , should be taking careful note at this stage. There is no documented datum that allows the use of concentrations to define an explicit amount, so, I'll make one up! I'll use Batch Volume (with Mash Thickness as an alternative ... perhaps?) to arrive at a representative figure in the style of home-brewing of a few years back. Perhaps this will be from a time whence came the nonsensical "the mash needs 50-150ppm calcium".
In my "parasitic spreadsheet" this looks like:
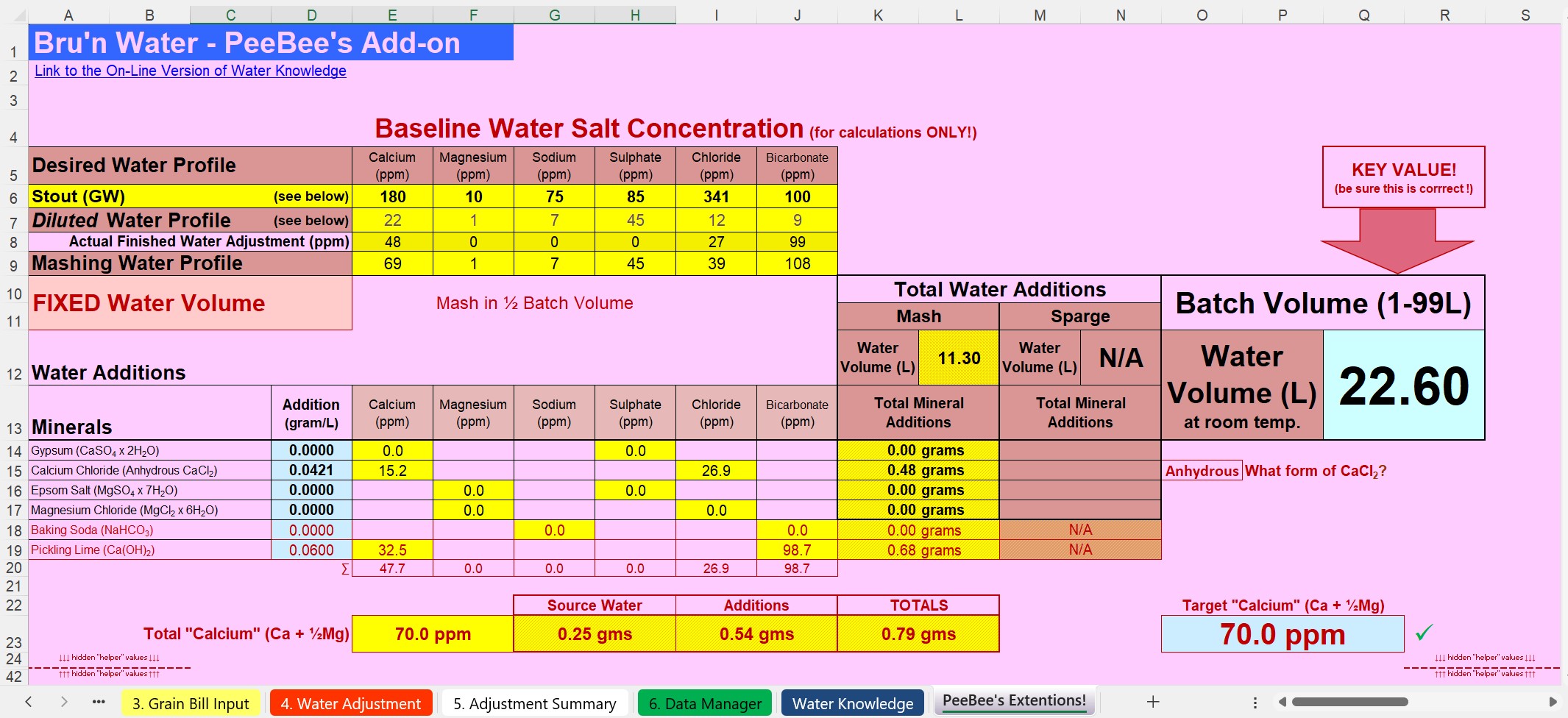
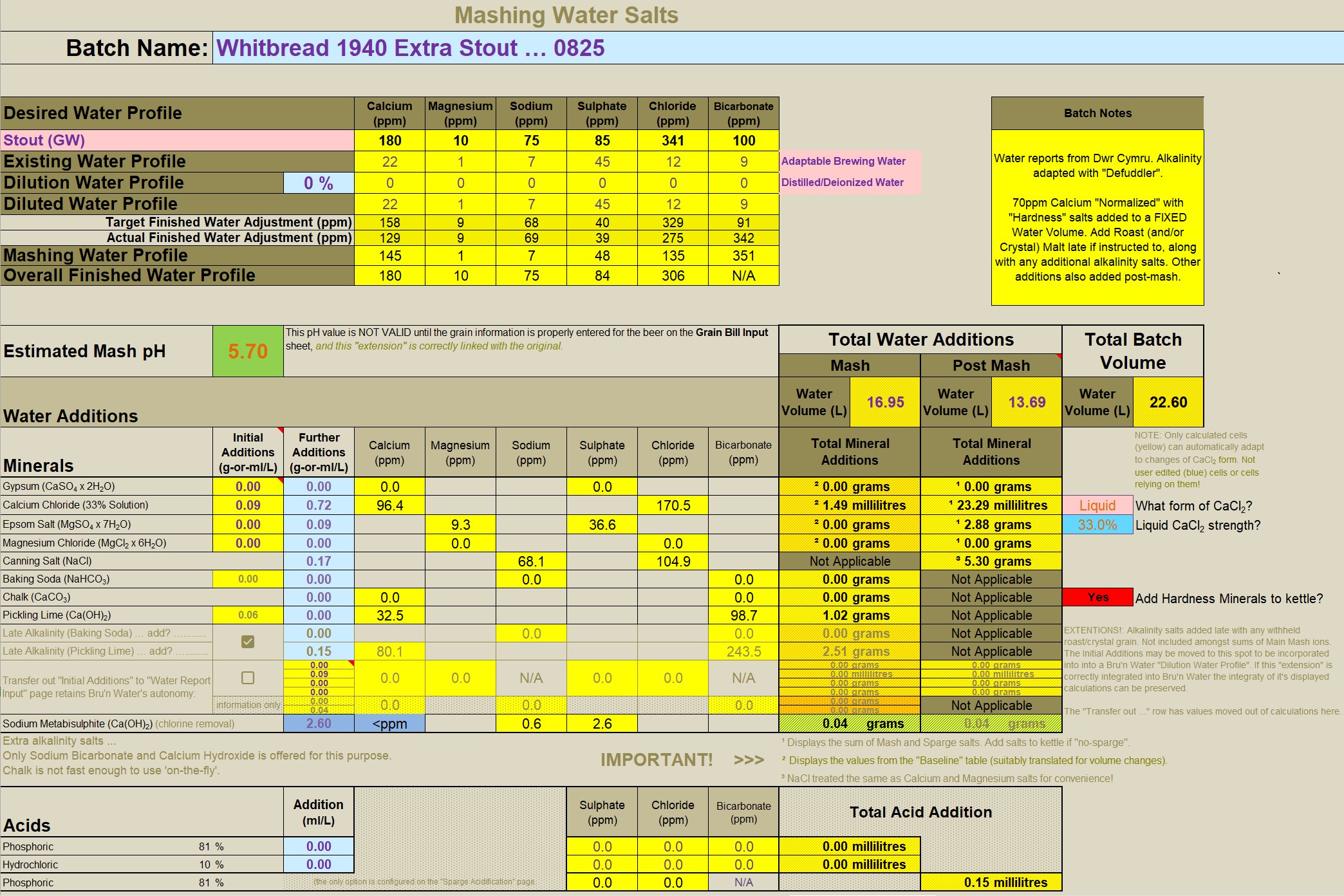
I've expanded out to show the well known tabs of Bru'n Water. This is as I said: Parasitic! But no devious hacks or modifications to Brun' Water. Bubblegum Pink background seemed appropiate. This is a fraction of the entire spreadsheet. It replicates the relevant chunk of Bru'n Water (it's an abbreviation of the Bru'n Water section).
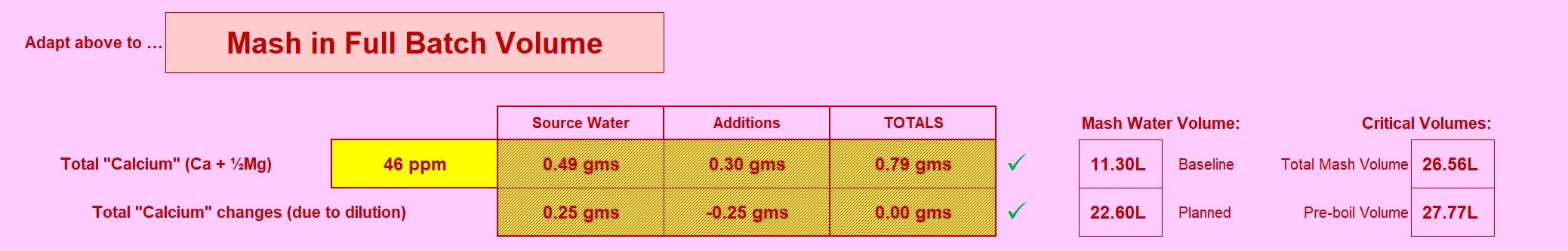
Using a Graham Wheeler's "Stout" profile, which is hefty enough to make a fairly challanging example. I'm setting the baseline at 70ppm of calcium (actually includes the tiny fraction of Magnesium "as Calcium"). 70ppm is about as high as the baseline should go: There is already alkalinity encrouching (as Calcium Hydroxide) to balance out the Calcium addition.
An important scheme is being demonstrated here: Note the very low solutes for the mash? The mash water doesn't need any more. "Sulphates to enhance hops"? This is mashing, there isn't any hops yet! So why encumber the mash with "sulphates"? (Answer: It's a history thing, nothing to do with brewing today). We have the ability to customise mash water (using RO water for instance), sulphates and the like can come later. The mashing water only needs Calcium (and Magnesium) with alkalinity to balance it to arrive at a suitable mash pH (Bru'n Water is clocking pH5.62 for this one - a London Stout remember).
That.s enough for now. Quite enough for folk to start lining up to crucify me again. Hopefully I've covered enough detail this time to let people know I'm serious about getting this pH business fixed!
I've been hacking away at the subject for a while now; I've not given up! It was a rant about home-brewer's careless mixing of "concentration" units with explicit units and the chaos that's resulting (like people measuring salts to accuracies of a hundredth of a gram ... and thinking it's doing any good!). It's taken me a while to collect enough to offer something more reasonable ... and that's because the errors are so deep ingrained, I couldn't help but get confused myself. I had a reason to keep hacking at it, I was getting "impossibly" low mash pHs (below 5.0).
For assistance I was (mis)using a respected water calculator ... Bru'n Water. As I do not have permission from Martin Brungard yet (?) I've to stick to pictures. The "parasitic spreadsheet" I created is perhaps "too close to the line" (not just the look and feel) to duplicate publicly?
The first thing I attempt to do is create a "baseline". I need the baseline to calculate salt additions without using "concentration" units. Anyone who attempts to measure salts to "hundredths of a gram", "tenths of a gram" or even less "accuracy" for that matter , should be taking careful note at this stage. There is no documented datum that allows the use of concentrations to define an explicit amount, so, I'll make one up! I'll use Batch Volume (with Mash Thickness as an alternative ... perhaps?) to arrive at a representative figure in the style of home-brewing of a few years back. Perhaps this will be from a time whence came the nonsensical "the mash needs 50-150ppm calcium".
In my "parasitic spreadsheet" this looks like:

I've expanded out to show the well known tabs of Bru'n Water. This is as I said: Parasitic! But no devious hacks or modifications to Brun' Water. Bubblegum Pink background seemed appropiate. This is a fraction of the entire spreadsheet. It replicates the relevant chunk of Bru'n Water (it's an abbreviation of the Bru'n Water section).
Using a Graham Wheeler's "Stout" profile, which is hefty enough to make a fairly challanging example. I'm setting the baseline at 70ppm of calcium (actually includes the tiny fraction of Magnesium "as Calcium"). 70ppm is about as high as the baseline should go: There is already alkalinity encrouching (as Calcium Hydroxide) to balance out the Calcium addition.
An important scheme is being demonstrated here: Note the very low solutes for the mash? The mash water doesn't need any more. "Sulphates to enhance hops"? This is mashing, there isn't any hops yet! So why encumber the mash with "sulphates"? (Answer: It's a history thing, nothing to do with brewing today). We have the ability to customise mash water (using RO water for instance), sulphates and the like can come later. The mashing water only needs Calcium (and Magnesium) with alkalinity to balance it to arrive at a suitable mash pH (Bru'n Water is clocking pH5.62 for this one - a London Stout remember).
That.s enough for now. Quite enough for folk to start lining up to crucify me again. Hopefully I've covered enough detail this time to let people know I'm serious about getting this pH business fixed!









![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)