wsmith1625
Well-Known Member
I'm trying to figure out how much 88% lactic acid to add to my brew water for a Cream of 3 Crops recipe I want to brew on Sunday. I've tried 3 water calculators and have 3 different answers to reach a target mash PH of 5.4. I'm shooting for a light lager water profile so I wasn't adding any additional minerals to the water.
Brewer's Friend says to add 7.01ml
BrunWater says to add 3.8ml
EZ Water Calculator says to add 3ml.
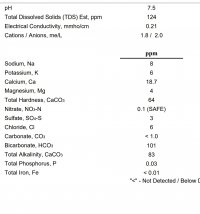
Here is my water profile (ppm) from Ward Labs
Calcium Ca - 18.7
Magnesium Mg - 4
Sodium Na - 8
Chloride Cl - 6
Sulfate SO4 - 9
Bicarbonate HCO3 - 101
Measured PH 7.5
Grain Bill
5.5 lbs. 2 Row 1.8
2.5 lbs. flaked corn .8
1 lb. instant rice 1
All 3 calculators don't really allow for flaked rice or flaked corn in the grain bill, so I was manually adding it and setting the color, .8 for the corn and 1 for the rice. I don't know if that's correct, but I found those numbers on the MoreBeer.com.
Brewer's Friend says to add 7.01ml
BrunWater says to add 3.8ml
EZ Water Calculator says to add 3ml.
Here is my water profile (ppm) from Ward Labs
Calcium Ca - 18.7
Magnesium Mg - 4
Sodium Na - 8
Chloride Cl - 6
Sulfate SO4 - 9
Bicarbonate HCO3 - 101
Measured PH 7.5
Grain Bill
5.5 lbs. 2 Row 1.8
2.5 lbs. flaked corn .8
1 lb. instant rice 1
All 3 calculators don't really allow for flaked rice or flaked corn in the grain bill, so I was manually adding it and setting the color, .8 for the corn and 1 for the rice. I don't know if that's correct, but I found those numbers on the MoreBeer.com.






















![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)