- Joined
- Jun 12, 2014
- Messages
- 574
- Reaction score
- 191
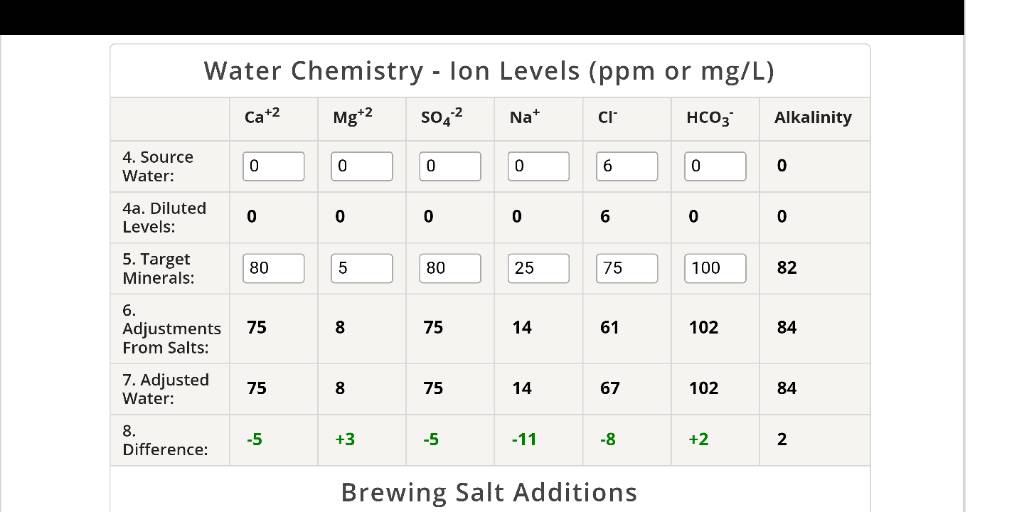
I tried doing water additions for the first time today. I used 16.5g water for my brew. 8.5 went in the mash. Rest went into the hlt for herms temp control and stuff.
Question I have.. the water looked milky after I added the minerals. And now that I've finished sparging my hlt is coated in white chalk. Is this normal?
I added
7g chalk
3g baking soda
5g gypsum
8g calcium chloride
5g Epsom salt
Question I have.. the water looked milky after I added the minerals. And now that I've finished sparging my hlt is coated in white chalk. Is this normal?
I added
7g chalk
3g baking soda
5g gypsum
8g calcium chloride
5g Epsom salt