Here's another one:
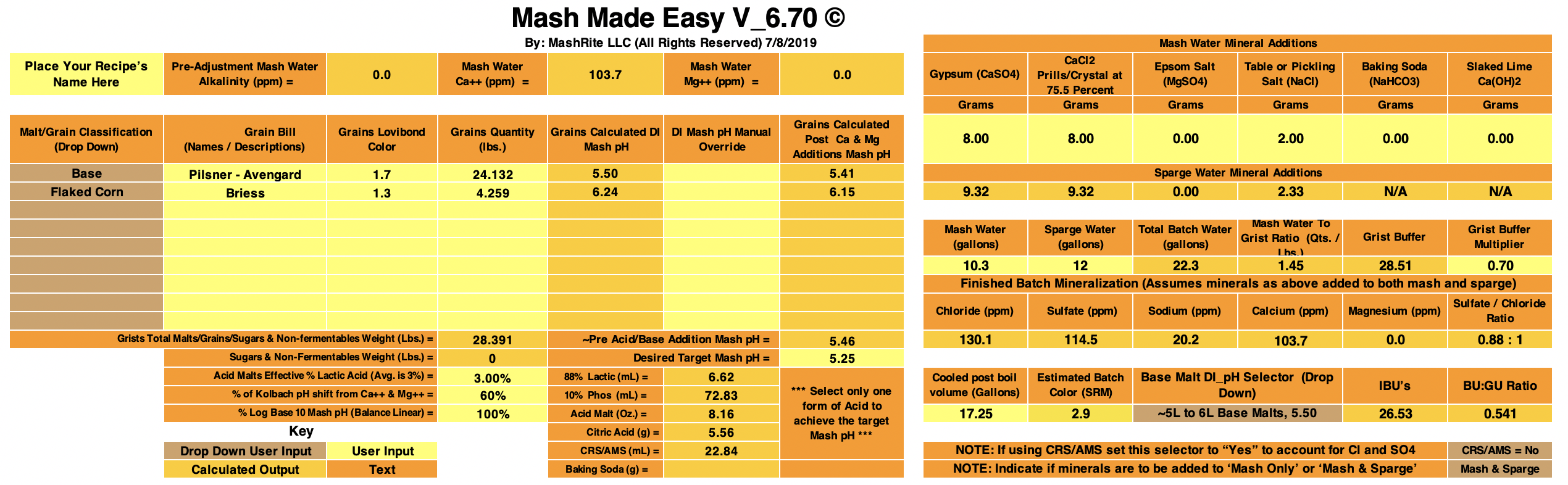
Pale Ale Recipe
4 lbs Maris Otter
0.5 lbs Munich
0.5 lbs Flaked Oats
0.25 lbs Caramel 40L
5.0 gallons of tap water (after 3-filter activated carbon filtration).
LaMotte water test says: 30 ppm Chloride, 0 ppm Sulfate, Total Alkalinity 80 ppm (as CaCO3), Total Hardness 70 ppm (as CaCO3), Ca ions 24 ppm, Mg ions 2.42 ppm, Residual Alkalinity 61.4 ppm (as CaCO3), Sodium 24.38 ppm
2.3 g Gypsum
3.7 g Epsom
1.3 g CaCl
0.3 g NaCl
1.4 oz (41 ml) of 10% Phosphoric Acid
Mashed @ 122F for 30 min, then heated to 156F
@ 0 min into 156F mash: pH=5.53 @ 25.0C
@ 15 min into 156F mash: pH=5.55 @ 21.4C
@ 30 min into 156F mash: pH = 5.56 @ 22.6C
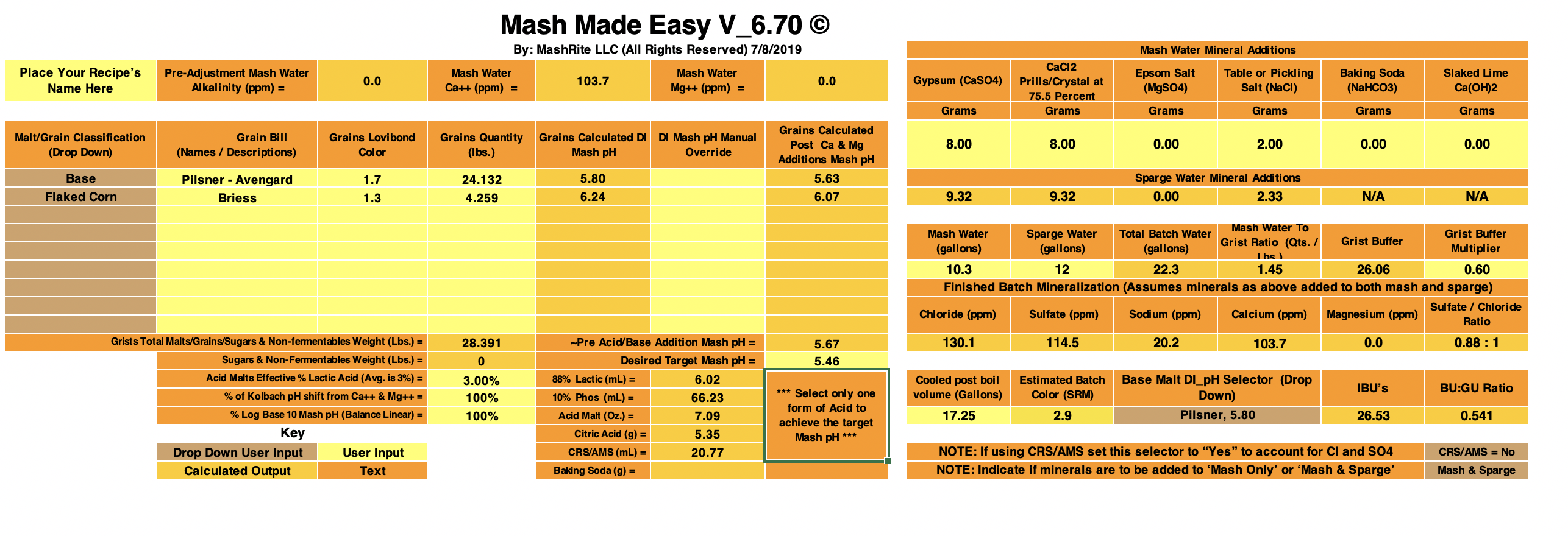
Pale Ale Recipe
4 lbs Maris Otter
0.5 lbs Munich
0.5 lbs Flaked Oats
0.25 lbs Caramel 40L
5.0 gallons of tap water (after 3-filter activated carbon filtration).
LaMotte water test says: 30 ppm Chloride, 0 ppm Sulfate, Total Alkalinity 80 ppm (as CaCO3), Total Hardness 70 ppm (as CaCO3), Ca ions 24 ppm, Mg ions 2.42 ppm, Residual Alkalinity 61.4 ppm (as CaCO3), Sodium 24.38 ppm
2.3 g Gypsum
3.7 g Epsom
1.3 g CaCl
0.3 g NaCl
1.4 oz (41 ml) of 10% Phosphoric Acid
Mashed @ 122F for 30 min, then heated to 156F
@ 0 min into 156F mash: pH=5.53 @ 25.0C
@ 15 min into 156F mash: pH=5.55 @ 21.4C
@ 30 min into 156F mash: pH = 5.56 @ 22.6C



































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)