Short story: I am requesting that actual mash pH measurements (and relevant ancillary information) be posted in this thread.
Motivation: A number of recent threads on this forum have been discussing the relative merits of various programs that predict mash pH. However, in order to assess and develop such programs, actual data is required. So far, such data appears to markedly absent. Hence this thread. I hope in the long run this thread will be a useful repository of data for anyone interested in this subject.
What's needed: The following is needed for such data to be useful.
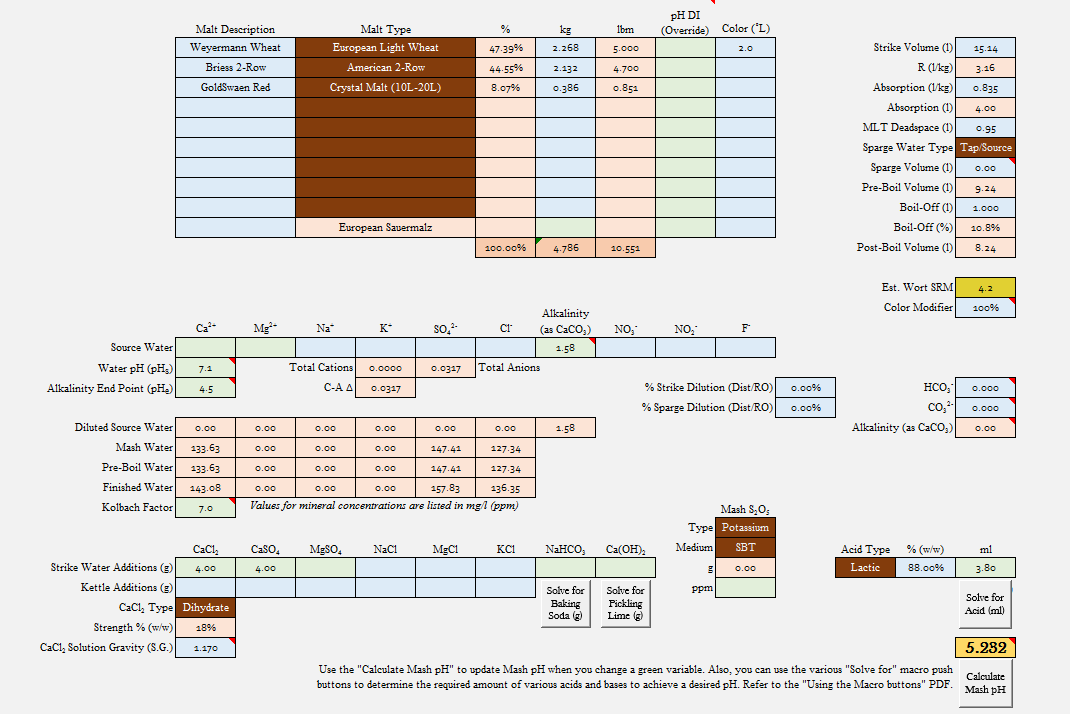
1. Grain bill [specific grains mashed and amount of each grain (in lbs), ideal if grain color (Lovibond) is also included)]
2. Amount of mash strike water (in gallons)
3. Ionic content of source water [Na, Ca, Mg, SO_4, Cl, Nitrate (if available) and alkalinity (as calcium carbonate or simply HCO_3 concentration), all in ppm (= mg/L)]
4. Salts added [CaCl, CaSO_4, MgSO_4, NaCl, NaHCO_3, and/or Ca(OH)_2, in grams]
5. Acids added [%'age and amount (in mL)]
6. Mash pH [when taken (from beginning of mash) and temperature of liquid when measured are helpful]
I'll get the ball rolling. Here are data from a recent beer of a fellow homebrewer:
5.0 Lbs Weyermann Pale Wheat
4.7 Lbs Briess American Brewer's Two-Row
0.85 Lbs Goldswaen Red (15L-23L caramel malt)
4 gallons distilled water
4 grams CaCl2
4 grams CaSO4
3.8 ml lactic acid (88%)
Mash pH 5.23 @ ~ 30 minutes
Cheers!