Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
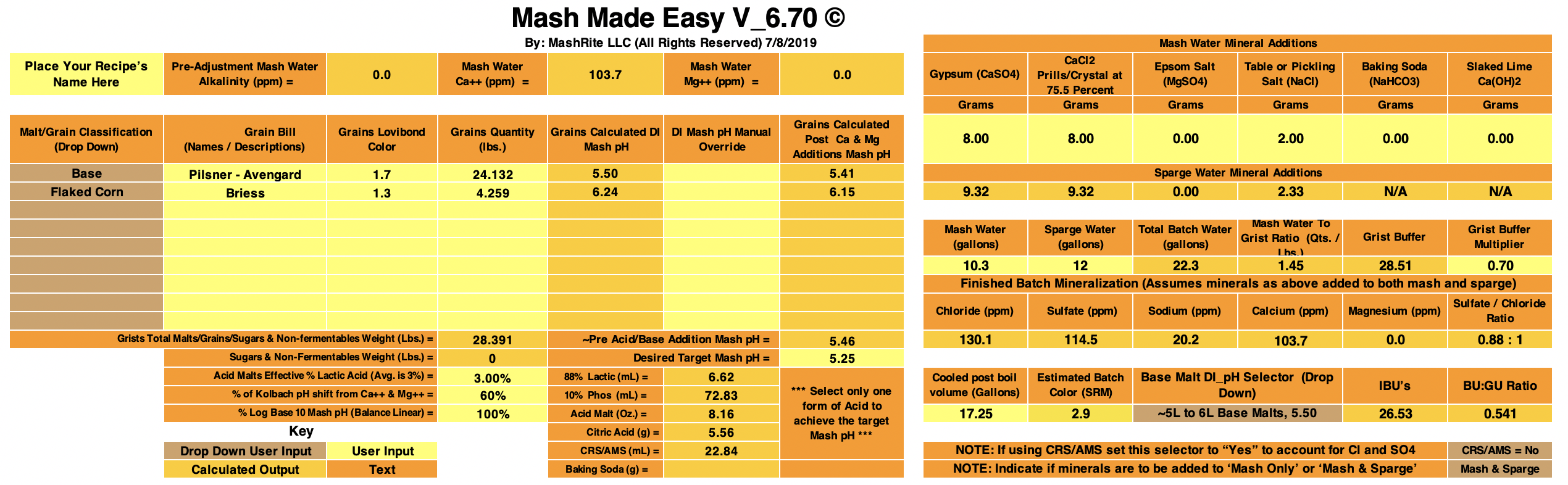
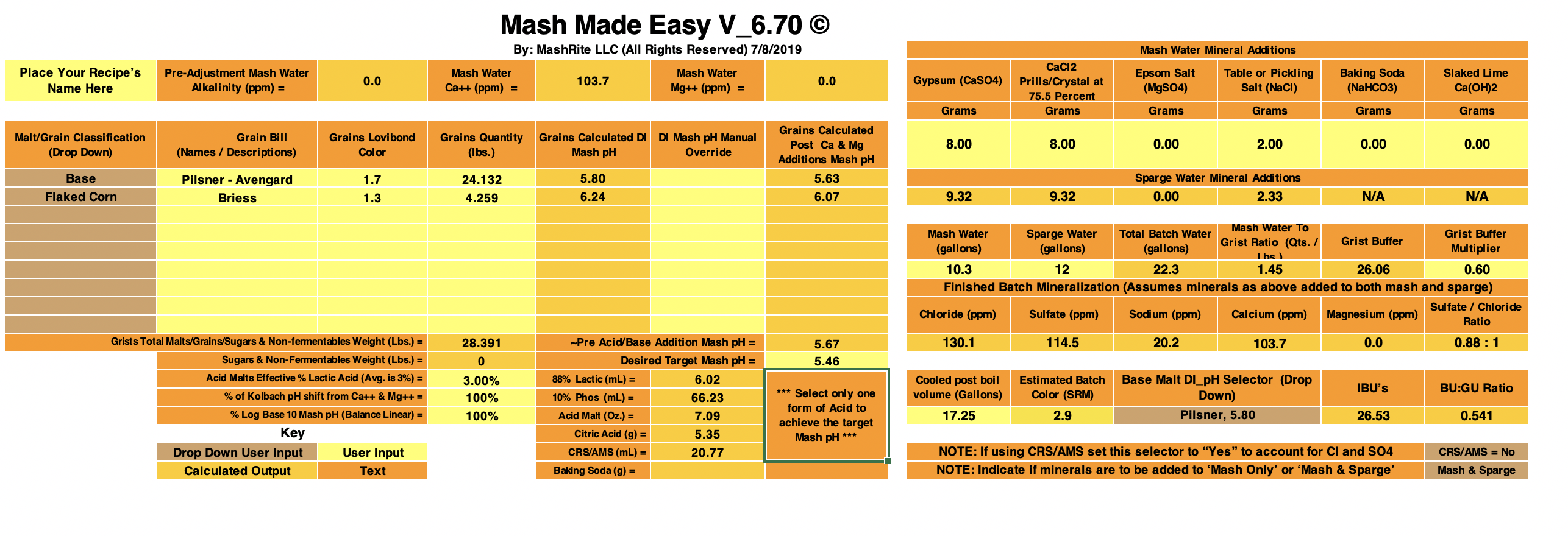
With Mash Made Easy version 6.00 (still in testing) I get 5.30 as the predicted mash pH for your Boont Amber Clone by accepting the initial defaults sans for selecting for Maris Otter.
MME is calling for an additional 1.49 grams of baking soda (on top of the 1.2 grams added) if you desire to hit 5.4 pH.

MME is calling for an additional 1.49 grams of baking soda (on top of the 1.2 grams added) if you desire to hit 5.4 pH.
Last edited: