Thesomeguy
Well-Known Member
Ok, so I have done a bunch of research and a lot of "google searches" and cant find a tried and true water profile for a blonde. I have received a report from ward labs for my water and discovered that for Mashing I need to Dilute the crap out of it. I will attach images of my water profile from Ward labs as well as screen shots of the Bru'n Water profile I am using.
I have read that you want to keep all ions low. Looking at the built profile for my blonde ale sadly I cant seem to bring my sulfate levels down anywhere near the desired profile levels so I shot to at least equal out my sulfate/Chloride ratio as I am looking for as balanced as I can get but not opposed to a hop forward flavor. I also am trying to get my calcium levels up which is why I added so much calcium Chloride. Now I know looking at the levels everything is green but I am not sure if that is appropriate for this beer so I am looking for input from the experts.
Thank you again for taking the time to read and comment on this, Water seems to have been one of the harder items for me to wrap my head around
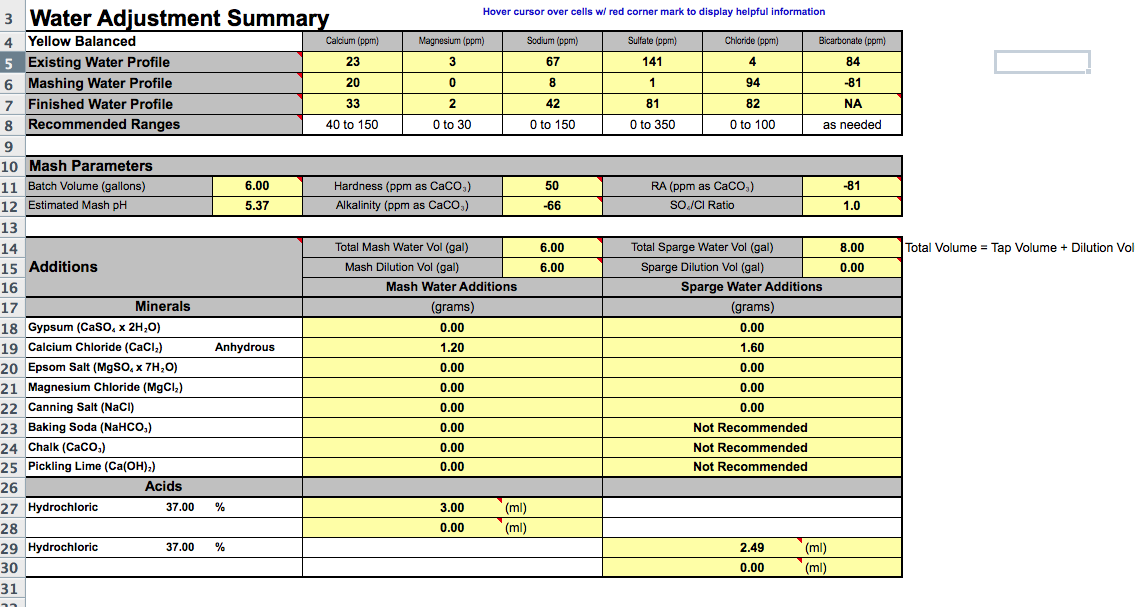
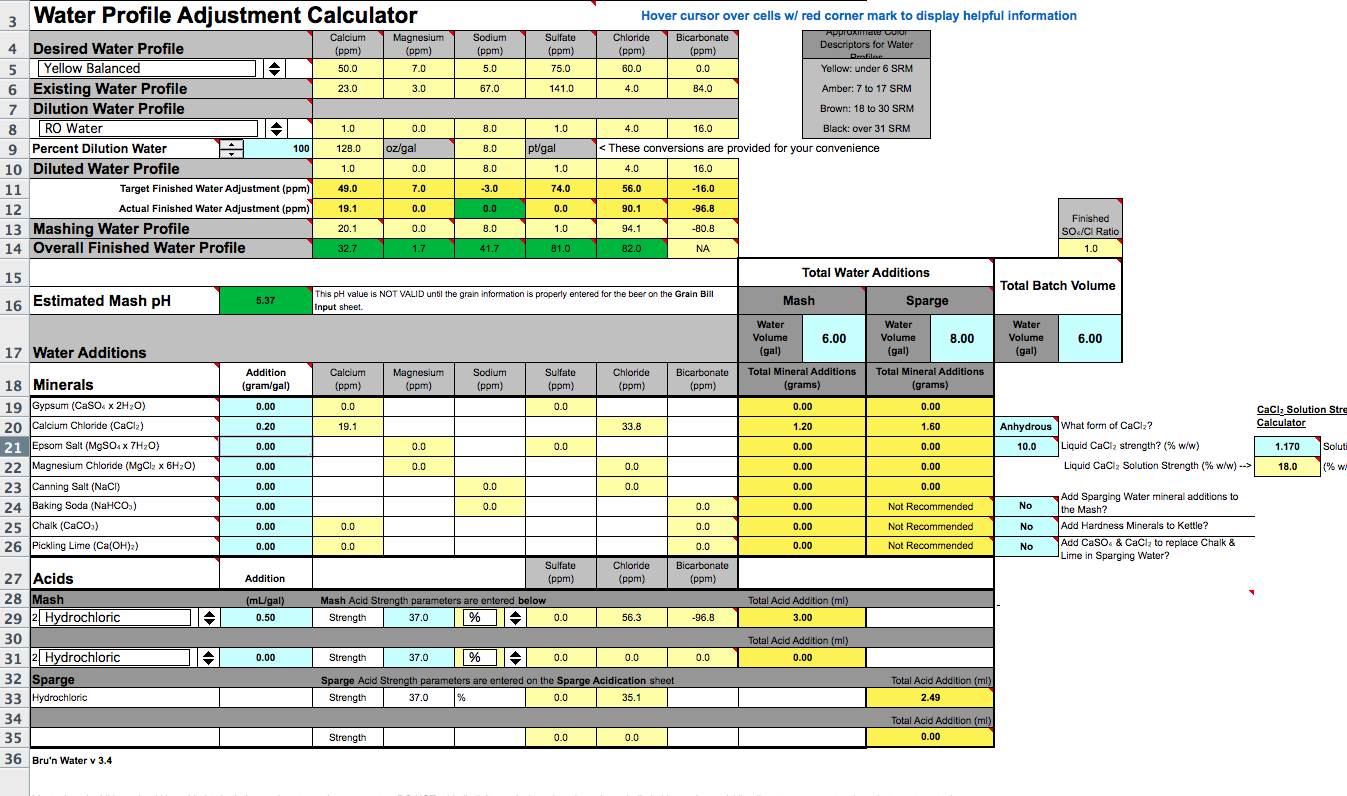
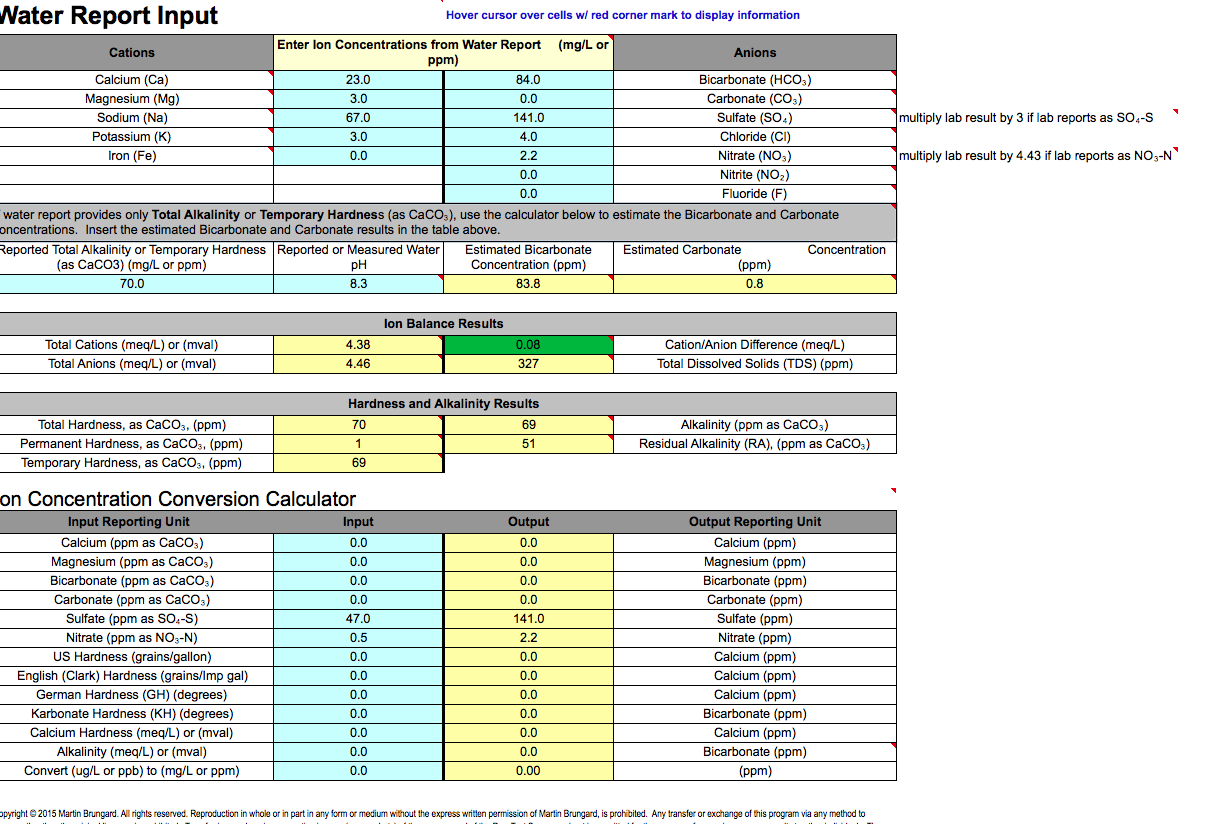

I have read that you want to keep all ions low. Looking at the built profile for my blonde ale sadly I cant seem to bring my sulfate levels down anywhere near the desired profile levels so I shot to at least equal out my sulfate/Chloride ratio as I am looking for as balanced as I can get but not opposed to a hop forward flavor. I also am trying to get my calcium levels up which is why I added so much calcium Chloride. Now I know looking at the levels everything is green but I am not sure if that is appropriate for this beer so I am looking for input from the experts.
Thank you again for taking the time to read and comment on this, Water seems to have been one of the harder items for me to wrap my head around











![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)































