my first batch of slants had this and I had not add yeast nutrients to these so I would have to say it was hot break.I get this too. I thought it was undissolved yeast nutrient but others here think that it is hot break. Either way it hasn't been an issue for me and I doubt it will be for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Slanting yeast
- Thread starter Saccharomyces
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
HollisBT
Well-Known Member
I figure that the yeast in a slant added to 10ml of wort and fermented out will have .8 billion cells. I posted about that, including where I got that information, earlier in this thread:
https://www.homebrewtalk.com/f163/slanting-yeast-133103/index59.html#post5779675
Thanks for the reply, but unfortunately the link just links back to the beginning of this thread for me... What is the post number?
Sent from my iPhone using Home Brew
HollisBT
Well-Known Member
Also, along he same lines as growing a pitch able amount of yeast from a slant...
How many steps (on average) does it take to grow a slurry with enough cells to pitch to a reasonable gravity beer? I have been playing with a few calculators online, and it seems like it would take about 4 to 5 steps to get enough cells to manage a roughly 1.060 brew. And with each step taking about 2 days (one day on the stir plate, and another day cool crashing) it seems like this method of yeast storage might be a little more involved than I was hoping...
Sent from my iPhone using Home Brew
How many steps (on average) does it take to grow a slurry with enough cells to pitch to a reasonable gravity beer? I have been playing with a few calculators online, and it seems like it would take about 4 to 5 steps to get enough cells to manage a roughly 1.060 brew. And with each step taking about 2 days (one day on the stir plate, and another day cool crashing) it seems like this method of yeast storage might be a little more involved than I was hoping...
Sent from my iPhone using Home Brew
tally350z
Well-Known Member
Its best to do the steps by 10 fold. So you would start out with a 5 or 10ml wort ---> 50 or 100ml ---> 500 or 1000ml, than one more to a another 1000ml or 1.5L. Since we have no way of actually knowing the cell count, I just go by the slurry volume..
HollisBT
Well-Known Member
Its best to do the steps by 10 fold. So you would start out with a 5 or 10ml wort ---> 50 or 100ml ---> 500 or 1000ml, than one more to a another 1000ml or 1.5L. Since we have no way of actually knowing the cell count, I just go by the slurry volume..
How do you estimate by slurry volume?
Sent from my iPhone using Home Brew
Also, along he same lines as growing a pitch able amount of yeast from a slant...
How many steps (on average) does it take to grow a slurry with enough cells to pitch to a reasonable gravity beer? I have been playing with a few calculators online, and it seems like it would take about 4 to 5 steps to get enough cells to manage a roughly 1.060 brew. And with each step taking about 2 days (one day on the stir plate, and another day cool crashing) it seems like this method of yeast storage might be a little more involved than I was hoping...
Sent from my iPhone using Home Brew
Don't cold crash as you are propagating. Pitch entire volume from one step to the next, and if you are using a stir plate, 24 hours seems to be fine between steps. I usually add 10 mls of sterile wort to my slant, and wait 24-48 hours for activity. From there I dump the entire contents into a media bottle with 90ml sterile wort (totaling 100ml) and a stir bar. This goes on the stir plate. 24 hours later there's plenty of yeasty activity going on and that whole volume gets added to a 1 Liter flask with 900mls of sterile wort. 24 hours later that volume gets added to a 2L flask with another 900mls of sterile wort. After 24 hours, pitch this whole volume or cold crash and decant. That should be plenty of yeast for a 1060 beer.
Sent from my Nexus 7 using Home Brew mobile app

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Gas MFL)
Guangshui Weilu You Trading Co., Ltd

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$76.92 ($2,179.04 / Ounce)
Brewing accessories 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304 Brewing accessories(Gas Hose Barb)
chuhanhandianzishangwu

$44.99
$49.95
Craft A Brew - Mead Making Kit – Reusable Make Your Own Mead Kit – Yields 1 Gallon of Mead
Craft a Brew

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$28.98
Five Star - 6022b_ - Star San - 32 Ounce - High Foaming Sanitizer
Great Fermentations of Indiana

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive

$719.00
$799.00
EdgeStar KC2000TWIN Full Size Dual Tap Kegerator & Draft Beer Dispenser - Black
Amazon.com

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$159.99 ($26.66 / Count)
3M High Flow Series System BREW120-MS, 5616001, For Brewed Coffee and Hot Tea, Valve-in-Head Design
SpaceCityProviders

$479.00
$559.00
EdgeStar KC1000SS Craft Brew Kegerator for 1/6 Barrel and Cornelius Kegs
Amazon.com
![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)
$6.95 ($17.38 / Ounce)
$7.47 ($18.68 / Ounce)
Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]
Hobby Homebrew
MichaelBrock
Well-Known Member
Thanks for the reply, but unfortunately the link just links back to the beginning of this thread for me... What is the post number?
Sent from my iPhone using Home Brew
The link does work for me. Might be a browser thing. The post number is 5779675. The article that I linked to there:
http://www.maltosefalcons.com/tech/yeast-propagation-and-maintenance-principles-and-practices\
does suggest an estimate of how many cells we are starting with. Also, contrary to the usual "10x steps" it suggests that we can use larger and fewer step-ups. I have been and it is working for me.
What is the consensus on the first step. I have read to use a loop and pull from the slant and inoculate a tube of sterile wort. I see it appears that others are putting sterile wort in the slant.
What is the consensus on the first step. I have read to use a loop and pull from the slant and inoculate a tube of sterile wort. I see it appears that others are putting sterile wort in the slant.
I like to use the whole slant because it cuts down on propagation time. If you want you can loop from a slant with yeast to a new slant before adding wort. This way you get a fresh new slant of yeast, and a higher starting cell count for your first step. I wouldn't do this for your last slant though, I would make sure you had more good slants of that strain before you use up the last slant of that specific strain.
Sent from my Nexus 7 using Home Brew mobile app
I like to use the whole slant because it cuts down on propagation time. If you want you can loop from a slant with yeast to a new slant before adding wort. This way you get a fresh new slant of yeast, and a higher starting cell count for your first step. I wouldn't do this for your last slant though, I would make sure you had more good slants of that strain before you use up the last slant of that specific strain.
Sent from my Nexus 7 using Home Brew mobile app
This seems like a good plan, as it keeps the yeast bank turned over.
ColoHox
Compulsive Hand Washer
What is the consensus on the first step. I have read to use a loop and pull from the slant and inoculate a tube of sterile wort. I see it appears that others are putting sterile wort in the slant.
It is preferable to pull a colony from the slant and inoculate the new 10ml culture as opposed to filling the slant with media. Pulling a colony or a few colonies and inoculating your new culture will maintain the purity of the culture, although it may be a bit slower to start.
This way, if you have a good slant, you can keep it for future use.
It is preferable to pull a colony from the slant and inoculate the new 10ml culture as opposed to filling the slant with media. Pulling a colony or a few colonies and inoculating your new culture will maintain the purity of the culture, although it may be a bit slower to start.
This way, if you have a good slant, you can keep it for future use.
I usually make 5 slants when I culture a new strain. When I'm down to 2 slants left of that strain, I'll inoculate 4 more slants. This keeps the culture active and alive. I don't wait til my last slant in case something happens, if #4 doesn't turn out, I still have my backup #5.
I'm not saying this is ideal, or even the proper way, but it fits my logic and has worked well for me.
Sent from my Galaxy Nexus using Home Brew mobile app
tally350z
Well-Known Member
You could make 4 or 5 tubes with media, and skip the agar so it is sterile media. That way you can measure out 10ml in each tube and autoclave it. Set them aside and when you are ready to propagate just take a loop and inoculate it in the sterile 10ml test tube. To take it a step further you can autoclave a small 500ml flask or beaker to get the next steps sterile and ready when you need them.
tally350z
Well-Known Member
How do you estimate by slurry volume?
Some use different measurement concentrations, but I use the 1 billion cells/per ml of slurry.
HollisBT
Well-Known Member
Don't cold crash as you are propagating. Pitch entire volume from one step to the next, and if you are using a stir plate, 24 hours seems to be fine between steps. I usually add 10 mls of sterile wort to my slant, and wait 24-48 hours for activity. From there I dump the entire contents into a media bottle with 90ml sterile wort (totaling 100ml) and a stir bar. This goes on the stir plate. 24 hours later there's plenty of yeasty activity going on and that whole volume gets added to a 1 Liter flask with 900mls of sterile wort. 24 hours later that volume gets added to a 2L flask with another 900mls of sterile wort. After 24 hours, pitch this whole volume or cold crash and decant. That should be plenty of yeast for a 1060 beer.
Sent from my Nexus 7 using Home Brew mobile app
So do you adjust the gravity for each dilution? Or do you just assume that the dilution will not effect the outcome enough to impact it?
Do you take any steps for counting cells? Or just assume that there will be enough in the 1 liter starter to healthily ferment your beer?
Sent from my iPhone using Home Brew
You could make 4 or 5 tubes with media, and skip the agar so it is sterile media. That way you can measure out 10ml in each tube and autoclave it. Set them aside and when you are ready to propagate just take a loop and inoculate it in the sterile 10ml test tube. To take it a step further you can autoclave a small 500ml flask or beaker to get the next steps sterile and ready when you need them.
I have 100ml culture bottles that I make wort in and run through my pressure cooker with multiple quart sized mason jars. I could do it the way you suggest, but this way I turn my slat cultures over more often and keep them relatively "fresh".
Sent from my Galaxy Nexus using Home Brew mobile app
So do you adjust the gravity for each dilution? Or do you just assume that the dilution will not effect the outcome enough to impact it?
Do you take any steps for counting cells? Or just assume that there will be enough in the 1 liter starter to healthily ferment your beer?
Sent from my iPhone using Home Brew
I don't take account for dilution, it will be minimal when you step up by5-10x the volume.
I don't do cell counts, but my experience has led me to believe that a 1.8 liter starter on a stir plate stepped up the way I explained earlier is enough yeast (and probably more then necessary) for a standard 5 gallon batch. For a beer with a greater than 1070 OG, I'll either make a 2.5-5 gallon batch of standard beer, then use the yeast cake to brew the bigger beer.
Sent from my Galaxy Nexus using Home Brew mobile app
tally350z
Well-Known Member
I have 100ml culture bottles that I make wort in and run through my pressure cooker with multiple quart sized mason jars. I could do it the way you suggest, but this way I turn my slat cultures over more often and keep them relatively "fresh".
Sent from my Galaxy Nexus using Home Brew mobile app
Do you fill the 100ml all the way or do you only fill them half way? So what you are saying is you begin the first propagation step by using 100ml? So when you are ready to propagate you transfer a loop to another slant and then propagate the slant you took the loop from? Is that my understanding of what your process is?
Do you fill the 100ml all the way or do you only fill them half way? So what you are saying is you begin the first propagation step by using 100ml? So when you are ready to propagate you transfer a loop to another slant and then propagate the slant you took the loop from? Is that my understanding of what your process is?
I start by taking 10ml out of the 100 with a sterile syringe and adding it to the slant. If I'm down to the last 2 slants, I'll use a loop to transfer from slant to slant before adding the sterile wort. After visible signs of fermentation start in the slant with wort, I transfer into the 100ml (I put stir bars in them before I pressure cook them). This then goes on a stir plate for 24-36 hours. Then up to a 1000ml flask with 900ml of sterile wort (from the mason jars I pre-pressure cook) for another 24-36 hours. At this point I'll either make a 2.5 gallon batch or step up to a 2liter flask, depending on what my plans are. I'll go 2.5 gallons then use that cake for a 10 gallon batch, of 2 liter starter on stir plate to 5 gallon batch. From there I'll use the yeast for a couple generations before ending that strain.
Sent from my Nexus 7 using Home Brew mobile app
tally350z
Well-Known Member
Oh ok I see now..when you add the fresh wort to the slant does it break up the streak enough for all the yeast to come out when you transfer it to the 90ml? Im assuming you shake the crap out of the slant with the wort?
How much yeast do you estimate you have after your 100mL or 1L process?
Also, I like the media jars... any particular brand/model work well? Also, what slant vials do you use?
Also, I like the media jars... any particular brand/model work well? Also, what slant vials do you use?
I use the vials the OP suggested, and the 100ml media bottles from the same company.
Since I don't have a microscope or a hemocytometer I don't bother guesstimating cell count. All guesses are wildly off. From experience, this process works for me. And works well.
Sent from my Nexus 7 using Home Brew mobile app
Since I don't have a microscope or a hemocytometer I don't bother guesstimating cell count. All guesses are wildly off. From experience, this process works for me. And works well.
Sent from my Nexus 7 using Home Brew mobile app
How much yeast do you estimate you have after your 100mL or 1L process?
Also, I like the media jars... any particular brand/model work well? Also, what slant vials do you use?
I recently wrote the article below thinking of posting it somewhere when I came across this thread. You will notice I am a purest when it come to yeast propagation so my methods are somewhat different from those previously described. That said, I believe those methods are sound.
Adventures in Yeast Propagation
For the past 6 years I have been propagating my own yeast from storage and using guesswork for pitching rates. I have assumed that a yeast culture propagated from a slant would have approximately the same cell density as a starter prepared from a liquid yeast pack. I ferment 10.5 gallon batches so I have wanted to use a 3 - 4 L culture in order to produce about 500 600 billion cells for my Belgian Strong ales but have been limited to a 1.5 L culture in my 2 L flask and cheap stir plate. Actually all was going well and my beers have won a lot of medals at some big shows. However, always wanting to brew better beer and believing I was under pitching, I purchased a 5 L flask, built a monster stir plate and began using a 4 L culture. To my surprise, the beer suffered a loss of aroma and flavor (fruity esters and spicy phenols) as reflected in my score sheets. Recently, I purchased a microscope and equipment to count cells. After much study and practice using a hemocytometer and making precise dilutions, I embarked on producing a 4 L culture from 5 single colonies on a streak plate and counted the cells at initial inoculations and at each increase. I want to share these results for those of you who are culturing yeast and want to have a better idea about yeast development but have not yet made the plunge into the microscopic world.
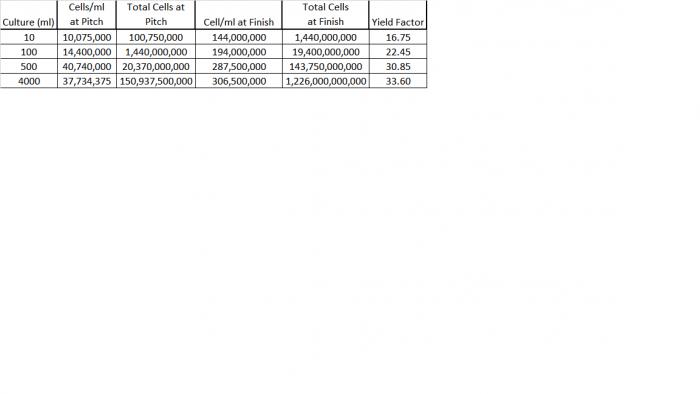
Five single colonies approximately 2mm in diameter were selected from a streak plate that had been incubated at 77° F for 96 hours and placed into a 50ml flask containing 10ml of 1.040 wort and a stir bar. The cell count after inoculation was 10,075,000/ml which could be calculated as 100,750,000 cells as the initial pitch. After 24 hours incubation on a stir plate at the same ambient temperature setting the cell count had increased to 144,000,000/ml with a total of 1.44 billion cells. The 10ml culture was transferred to 100ml of wort in a 250ml flask resulting in a calculated cell density of 14,400,000/ml. After 24 hours under the same conditions the cell count was 194,000,000/ml and calculated as 19.4 billion cells. This was transferred to 500ml of wort in a 1000ml flask resulting in a calculated cell density of 40,740,000ml. After 24 hours I checked the temperature of the culture and found it at 82°F even though the ambient was still set at 77° F. Apparently the stir plate and/or fermentation activity was increasing the temp by 5° F. The cell count was 287,500,000/ml. So the total number of cells in the 500ml culture was 151 billion cells, 1.5 time the amount in a commercial liquid yeast pack. The 500ml culture was transferred to 3500ml of wort, the calculated cell density was 37,734,375/ml. The temperature of the culture was brought down slowly from 82° F to 70° F over the next 36 hours when the final cell count was taken the total was 306,500,000 cells/ml giving an total culture count of 1,287,300,000,000 or over 2.25 trillion cells. I ended up pitching 1600ml of the culture, back to near where I was before I bought the monster flask and stir plate, the wonderful Belgian yeast flavors and aromas are back.
Note: In order to achieve these cell counts, it is essential preform aerobic fermentation at all stages. So be sure to have good aeration. Do not use an airlock, do not overfill your flasks (75% of the total flask capacity is max and less is better). If possible, allow the whirlpool to reach all the way down to the stir bar.
Hoptimistic
Well-Known Member
WOW just wow. crazy how simple this is. What is that yellow stuff. and where can you get it.
tally350z
Well-Known Member
So because you used 5 single cell colonies, would you say that using a single colony would result in 1/5 of the total cell count at the end. I know that it is not necessarily linear like that but would that be safe to assume? That's some awesome information you posted there. Or since most people on here take a loop from a slant rather than a plate for propagation could you assume that the size of the loop taken from the slant would equal 5 single colonies that are 2mm in diameter, because the size of the loop I use is close to 3-4mm in diameter.
So because you used 5 single cell colonies, would you say that using a single colony would result in 1/5 of the total cell count at the end. I know that it is not necessarily linear like that but would that be safe to assume? That's some awesome information you posted there. Or since most people on here take a loop from a slant rather than a plate for propagation could you assume that the size of the loop taken from the slant would equal 5 single colonies that are 2mm in diameter, because the size of the loop I use is close to 3-4mm in diameter.
Yes, I think it would be close if the colonies were about the same size (2mm). But I have never had much luck in getting single cells on a slant, I usually get a cover of yeast (lawn) on my slants. If that's the case I would say take 4 or 5 loops. I think I also read some people flood the slant and use the entire amount, which would also be OK. For propagation higher inoculation rates results in a higher yield factor (see table below). I'm sure there is a point of diminishing return, perhaps it's about 38 million cells/ml. It has been suggested (Chris White I believe) that you should not use one single colony for propagation to ensure genetic diversity, makes sense.
My reason for doing a streak plate is to ensure I am getting clean yeast. If there is contamination in your slant, you my not see it, especially if it is bacterial. It adds some time to the process but not much work or expense. Also, less slants, I make up two slants of each of my isolates and use one as a working culture and keep one closed for storage. Every six months or so I re-isolate and re-slant from the storage slant.
@trentm What's your 'hardware' ?
Regarding cell counting:
Microscope - AmScope, model B100B-MS $195 with free shipping http://www.amscope.com/
Obviously this is a cheap microscope but it is adequate for cell counting. Make sure you get one with a mechanical stage and 400X capacity.
Items below were purchased from: Cynmar: http://www.cynmar.com
BRIGHT-LINED HEMACYTOMETER, Stock#: 01200150, $65
There are cheaper hemocytometers out their but beware. You get what you pay for.
PIPET, A100, DIGITAL, 10.0 to 100.0µl, TIP EJECTOR - Stock#: 13534606, $99
PIPET, A1000, DIGITAL, 100.0 to 1000.0µl, TIP EJECTOR-Stock#: 13534610 $99
MICROPIPET TIPS w/RACK, FITS TO 200.0µl, 96/PK - Stock#: 13534782, $7
MICROPIPET TIPS w/RACK, FITS TO 1000µl, 100/PK - Stock#: 13534784, $7
MICROCENTRIFUGE TUBES, 1.5ml, GRADUATED, BLUE, 500/PK
Stock#: 13223125 $10.15
MICROTUBE RACK, 20-WELL, 1.5-2.0ml, BLUE PP Stock#: 13225570 $5.25
The pipettors and accessories are for making dilutions which is necessary, however there are cheaper ways of making accurate dilutions. That said, I have not regretted spending the money on the pipettors.
helibrewer
Well-Known Member
Interesting stuff. White and Zainasheff's study on starter sizes from 500ml to 8L indicated that the maximum yield factor occurs from inoculation rates between 50-70 million/ml. Your data seems to be trending the same.
Interesting stuff. White and Zainasheff's study on starter sizes from 500ml to 8L indicated that the maximum yield factor occurs from inoculation rates between 50-70 million/ml. Your data seems to be trending the same.
Interesting observation. That study was done without any supplemental oxygen or agitation. Thus the low yield factor. Obviously the fermentation would soon go anaerobic, at that point, as White says, "you are making beer and not propagating yeast". It would be interesting to know how aerobic propagation affects inoculation rates.
HollisBT
Well-Known Member
If you take a look at braukaisers research, he suggest that inoculation rate alone is not exactly enough information.
His research shows a trend that links the inoculation rate as well as how much food the culture is given.
This theory makes sense to me, but what doesn't exactly seem legit in my opinion is how dramatic his cell growth counts are. He is drastically higher than whites numbers show.
Sent from my iPhone using Home Brew
His research shows a trend that links the inoculation rate as well as how much food the culture is given.
This theory makes sense to me, but what doesn't exactly seem legit in my opinion is how dramatic his cell growth counts are. He is drastically higher than whites numbers show.
Sent from my iPhone using Home Brew
I recently wrote the article below thinking of posting it somewhere when I came across this thread. You will notice I am a purest when it come to yeast propagation so my methods are somewhat different from those previously described. That said, I believe those methods are sound.
Adventures in Yeast Propagation
Thanks for all good information's.
tally350z
Well-Known Member
Here is my first attempt at plating. I guess the only thing I can think of is to spread it out a little more.
Thoughts?

Thoughts?

HollisBT
Well-Known Member
During propagation, how bad is it if you absolutely HAVE to cold crash a starter?
For example, playing around with a few calculators... If I am stepping started up to a point where I need to make a 1.5l starter, and then repeat with another 1.5l starter.
Would I be better off splitting it into two flasks, and then adding a higher gravity wort to account for dilution? Or can I simply cold crash, decant, and then add more 1.040 wort on top and return to the stir plate?
For example, playing around with a few calculators... If I am stepping started up to a point where I need to make a 1.5l starter, and then repeat with another 1.5l starter.
Would I be better off splitting it into two flasks, and then adding a higher gravity wort to account for dilution? Or can I simply cold crash, decant, and then add more 1.040 wort on top and return to the stir plate?
tally350z
Well-Known Member
During propagation, how bad is it if you absolutely HAVE to cold crash a starter?
For example, playing around with a few calculators... If I am stepping started up to a point where I need to make a 1.5l starter, and then repeat with another 1.5l starter.
Would I be better off splitting it into two flasks, and then adding a higher gravity wort to account for dilution? Or can I simply cold crash, decant, and then add more 1.040 wort on top and return to the stir plate?
Best thing to do is to make your 1.5L starter, cold crash, decant, than make your second 1.5L starter and add right on top.
tally350z
Well-Known Member
So when I take the plate and am ready to transfer to the slant, should I take a single cell mound or should I combine two or three individual single cell mounds? I am thinking only one that way it is a single cell colony, I just want to make sure before transfer.
So when I take the plate and am ready to transfer to the slant, should I take a single cell mound or should I combine two or three individual single cell mounds? I am thinking only one that way it is a single cell colony, I just want to make sure before transfer.
In my view you are right to store from a single colony. It is a good idea to evaluate the colonies to try select one that is of average size and appearance with no apparent contamination. Smaller colonies could be mutants and the larger colonies may have developed from more than one cell. Fungal contamination is obvious while bacterial is hard to see but may appear shinny or form a halo around the colony.
Remember, it only take a tiny amount of the colony to inoculate the slant. If is visible on the loop you have plenty.
When selecting colonies grown up on a plate from storage for propagation it is then a good idea to select a few (I try to use 5) colonies to ensure genetic diversity as it is possible for yeast cells to develop genetic drift during storage.
NaymzJaymz
Well-Known Member
My slants have been incubating for ten days now. The yeast growth started quickly, but never advanced to the level of the slant in the authors picture. Is this due to the type of yeast (WLP060)? Any thoughts? Thank you!!!!
Similar threads
- Replies
- 8
- Views
- 3K
- Replies
- 3
- Views
- 743
- Replies
- 17
- Views
- 2K











































