You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Slanting yeast
- Thread starter Saccharomyces
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
EarlyAmateurZymurgist
Well-Known Member
- Joined
- Aug 4, 2013
- Messages
- 518
- Reaction score
- 140
I won't rehash my previous questions other than to say I've followed the directions implicitly and produced 12 blank slants which were being stored in a sterilized ziplock bag with lids tight and electrical tape sealing. The unused slants were made about 2 months ago. Upon examining them today I noticed one slant was completely corrupted by green mold. A couple of others had a light film of mold. Any ideas what went wrong? I actually pressure cooked the mixture filled vials a little longer than the directions implied. Where was the mistake? Should I assume the good looking slants are still okay? If they were supposedly sterile they should have lasted indefinitely, even if they hadn't been inoculated yet. I don't see how the slants could have been contaminated during the brief moments of lifting them out of the pressure cooker, tightening the lids, and wrapping them with the tape. Thank you!
My suggestion is to purchase a roll of autoclave tape. Autoclave tap looks a lot like plain old masking tape before being placed into a commercial autoclave or a home pressure cooker. Black diagonal stripes appear on the tape when sterilization temperatures are achieved. Autoclave tape doesn't indicate sterility. It merely indicates that the tape was exposed to a moist heat level that was high enough to cause sterilization to occur.
Autoclave Tape Post Processing

I prefer to use Parafilm to seal my plates and slants. Parafilm is like a stretchable wax coated cellophane wrap (it's actually polyolefin). One can use laboratory Parafilm M or Parafilm Grafting tape to seal slants and plates (Parafilm Grafting tape has a little more polyolefin and a little less wax). Neither product is sterile, so I usually spray both sides of the wrap with 91% isopropyl alcohol and allow it to flash off before sealing a slant or a plate.
My Latest Bank

Finally gave this a try over the weekend.
I got my agar and a few tubes from morebeer, since I was making an order anyway.
First attempt, total failure. I used the mix ratio on Kia's page, and it got very thick, very quick. At boiling it was yogurt thick.
Went through the process anyhow.:smack: Sparing the details, it was a horrible mess.

Second attempt, I started with 200 ml of 9ºP cool wort added 3g agar for a 1.5% mix, let it bloom for 15-20 min. then brought it up to a simmer.
It was still rather thick, I had to keep the heat on it to stay liquid enough to syringe it into my tubes.
I pulled them pretty soon after pressure dropped on the canner to lay them into position, and the consistency was similar to honey.
I also poured a couple of plates with the extra.
End result, they look good. Guess I'll see in a few days if they are clean and good to use.
I got my agar and a few tubes from morebeer, since I was making an order anyway.
First attempt, total failure. I used the mix ratio on Kia's page, and it got very thick, very quick. At boiling it was yogurt thick.
Went through the process anyhow.:smack: Sparing the details, it was a horrible mess.
Second attempt, I started with 200 ml of 9ºP cool wort added 3g agar for a 1.5% mix, let it bloom for 15-20 min. then brought it up to a simmer.
It was still rather thick, I had to keep the heat on it to stay liquid enough to syringe it into my tubes.
I pulled them pretty soon after pressure dropped on the canner to lay them into position, and the consistency was similar to honey.
I also poured a couple of plates with the extra.
End result, they look good. Guess I'll see in a few days if they are clean and good to use.
tally350z
Well-Known Member
How many of you that use slants also use plates?
brewski09
Well-Known Member
How many of you that use slants also use plates?
Technically, if you slant you should be plating first to assure a pure single colony was used. I plated before I slanted.
Sent from my Nexus 7 using Home Brew mobile app
tally350z
Well-Known Member
Technically, if you slant you should be plating first to assure a pure single colony was used. I plated before I slanted.
Yea, thats what I plan on starting with. I just really havn't seen too many posts of people using the plates. So what is your technique. My plan is to get the fresh yeast vial, steak onto a plate and incubate that. Then when needed transfer to a slant and grow up that until ready to create my starter? Does that sound about accurate?

$28.98
Five Star - 6022b_ - Star San - 32 Ounce - High Foaming Sanitizer
Great Fermentations of Indiana

$719.00
$799.00
EdgeStar KC2000TWIN Full Size Dual Tap Kegerator & Draft Beer Dispenser - Black
Amazon.com

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Gas MFL)
Guangshui Weilu You Trading Co., Ltd

$159.99 ($26.66 / Count)
3M High Flow Series System BREW120-MS, 5616001, For Brewed Coffee and Hot Tea, Valve-in-Head Design
SpaceCityProviders

$76.92 ($2,179.04 / Ounce)
Brewing accessories 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304 Brewing accessories(Gas Hose Barb)
chuhanhandianzishangwu
![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)
$6.95 ($17.38 / Ounce)
$7.47 ($18.68 / Ounce)
Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]
Hobby Homebrew

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$479.00
$559.00
EdgeStar KC1000SS Craft Brew Kegerator for 1/6 Barrel and Cornelius Kegs
Amazon.com

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$44.99
$49.95
Craft A Brew - Mead Making Kit – Reusable Make Your Own Mead Kit – Yields 1 Gallon of Mead
Craft a Brew

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi
brewski09
Well-Known Member
Yea, thats what I plan on starting with. I just really havn't seen too many posts of people using the plates. So what is your technique. My plan is to get the fresh yeast vial, steak onto a plate and incubate that. Then when needed transfer to a slant and grow up that until ready to create my starter? Does that sound about accurate?
I plated and made slants for storage. The plate is fine short term, but I think the vials are better for longer term storage.
Sent from my Nexus 7 using Home Brew mobile app
tally350z
Well-Known Member
So I am attempting to make plates. Trying to just get down the right process before I actually start plating yeast.
How thick should the plate media be? Haven't really been able to find a proper answer. I poured maybe 20-30ml into the plate. Should I try to make it as thin as possible?
How thick should the plate media be? Haven't really been able to find a proper answer. I poured maybe 20-30ml into the plate. Should I try to make it as thin as possible?
going to try and plate some Conan yeast that i harvested last fall, fermented with, and then washed. I made the plates last weekend.
On another note, i have attempted to purchase some culture tubes from Midland Scientific, as Zymurgist suggested in post #583 and it appears that they do not sell them individually. Smallest quantity appears to be 144 for $285.
On another note, i have attempted to purchase some culture tubes from Midland Scientific, as Zymurgist suggested in post #583 and it appears that they do not sell them individually. Smallest quantity appears to be 144 for $285.
Strike that! I have been emailing them today. They do not have the corning tubes, but they do have the Kimble tubes by the each. Under $2 ea.
I guess when i made my agar media i must not have added enough agar. When i ran my needle through them it apparently hadn't completely set up, cut portions would slide down when i held the plate vertically. So we'll see what grows in a day or two!!
ColoHox
Compulsive Hand Washer
So I am attempting to make plates. Trying to just get down the right process before I actually start plating yeast.
How thick should the plate media be? Haven't really been able to find a proper answer. I poured maybe 20-30ml into the plate. Should I try to make it as thin as possible?
There is not a hard rule as to the volume. The plate is normally filled 1/2 - 3/4 full. Thinly poured plates can dry out and crack, which makes harvest difficult.
NaymzJaymz
Well-Known Member
Thank you, Sir. One previousl replier stated that if I didn't achieve 15psi, that may be the reason. The instructions state that 12psi is what he used. I'm sure my bargain pressure cooker achieved 12psi, even though there is no gauge. Do you believe that I contaminated some of the slants when I picked them up and sealed the vials with electrical tape, or was the tape itself to blame? Is autoclave tape easily available? Once again I need to ask if you think the slants that "look" okay are okay to use. If there was bacteria present, why did it take so long for the contaminated slants to look infected? How do you use autoclave tape in this application? Obviously, you cant screw the caps down tight and seal them with autoclave tape before you pressure cook them, right? I can't see how to post photos on this site, or I would do so. Thank you!!!!
tally350z
Well-Known Member
Alright the first test run of making plates was a semi-success. After I poured in the media into the hot plate I covered and let cool and set. After they set I have read that you should turn them upside down to get rid of the condensation that is built up on the covers. I did this and then after a few days I flipped them back over and that is where I think I ran into the problem. Two of the 4 have grown some funkyness, the other two are doing ok for now.
Is that common practice to get rid of the condensation. Or am just not pouring the media hot/fast enough?
Is that common practice to get rid of the condensation. Or am just not pouring the media hot/fast enough?
ColoHox
Compulsive Hand Washer
Alright the first test run of making plates was a semi-success. After I poured in the media into the hot plate I covered and let cool and set. After they set I have read that you should turn them upside down to get rid of the condensation that is built up on the covers. I did this and then after a few days I flipped them back over and that is where I think I ran into the problem. Two of the 4 have grown some funkyness, the other two are doing ok for now.
Is that common practice to get rid of the condensation. Or am just not pouring the media hot/fast enough?
Plates are usually stored upside down, both before and after inoculation. Condensation will develop when you move them between temperatures and it is best to have it form on the lid.
The condensation can be a source of contaminating organisms, but it would be hard to say where yours came from.
EarlyAmateurZymurgist
Well-Known Member
- Joined
- Aug 4, 2013
- Messages
- 518
- Reaction score
- 140
Alright the first test run of making plates was a semi-success. After I poured in the media into the hot plate I covered and let cool and set. After they set I have read that you should turn them upside down to get rid of the condensation that is built up on the covers. I did this and then after a few days I flipped them back over and that is where I think I ran into the problem. Two of the 4 have grown some funkyness, the other two are doing ok for now.
Is that common practice to get rid of the condensation. Or am just not pouring the media hot/fast enough?
If large droplets are forming on your lids after pouring, then your dishes are too cool, media is too hot, and/or you are using too much media per plate. I only pour enough media to cover the bottom of the dish to a depth of approximately a 1.5 to 2 millimeters. If you time things correctly, only a small amount of condensation will form on the lids.
Here's what my plates look like after pouring:

I always proof my plates with the lid up before sealing them with Parafilm and storing them upside down in a sealed container in my brewing refrigerator. Depending on my schedule, I proof for two to three days. In my experience, proofing plates with the lids down in a home environment is a recipe for contamination, as dust particles can get between the bottom and the cover of the dish (the average home has significantly more airborne house dust than the average microbiology lab). These particles will find their way onto the media when the plate is turned over for streaking. House dust is a rich source of wild microflora.
As I mentioned above, very little condensation will form on the lids if you time things correctly and pour just enough media to cover the bottom of the dish to between 1.5 and 2 millimeters. This condensation will evaporate during the proofing period. The media will also dry out a little bit, which makes streaking a plate without tearing the surface of the media much easier. As long as your proofed plates are stored upside down and sealed tightly with Parafilm, you should experience very little evaporation during storage. I experience zero media cracking or lifting on my plates while in storage, and I store my plates up to six months at a time (I use them within that period of time).
EarlyAmateurZymurgist
Well-Known Member
- Joined
- Aug 4, 2013
- Messages
- 518
- Reaction score
- 140
How many of you that use slants also use plates?
While I routinely subculture slants directly from other slants, I always plate a liquid culture for "singles" before slanting it. When one plates a culture for singles and inoculates multiple slants each with a single colony from the plate, one effectively ends up with multiple isolates that may or may not behave the same. The difference in performance should be minimal when plating a single strain culture (real Ringwood produces isolates that do not behave the same because it contains multiple strains of yeast). What I like to do sometimes is to create several isolates and run test batches. I select the best performing isolate as my reference culture for the strain and make it my master slant.
tally350z
Well-Known Member
I guess I need to pick up some Parafilm. I think thats where the infection came from. When I turned them upside down they weren't seal properly so particles got in.
So would the best practice to start be to... plate the yeast >>> get single cell and transfer that to slant. >>> than transfer from that slant to other slant to ensure same yeast colony?
So would the best practice to start be to... plate the yeast >>> get single cell and transfer that to slant. >>> than transfer from that slant to other slant to ensure same yeast colony?
MichaelBrock
Well-Known Member
That probably is the "best practice". EarlyAmateurZymurgist undoubtedly practices best practice. I don't think EarlyAmateurZymurgist trusts the purity of commercial yeast producers. However, most of us create our slants directly from the yeast vial and haven't ever had an issue.
EarlyAmateurZymurgist
Well-Known Member
- Joined
- Aug 4, 2013
- Messages
- 518
- Reaction score
- 140
Yes, that is the process to follow. All slants that are inoculated from the initial slant will contain the descendants of the single yeast cell that formed the colony on the plate. If one is planning to use a culture several times within the period of a year, one should make working slants from the master. If one is planning to use a culture once every six months to year, one should plan on making a new master slant when one propagates a culture for brewing. Slanted cultures may experience drift over time, but that is not necessarily a bad thing. The only way to prevent (or greatly reduce) genetic drift is deep cryogenic storage. We are talking about temperatures that are much colder than a home deep freezer.
By the way, I always proof and incubate my plates with the cover up. The only time that I store my plates upside down is while they are in my brewing refrigerator (I seal my plates with Parafilm before storing them in my brewing refrigerator). Storing plates upside down in a refrigerator prevents condensation from forming in their lids. It also helps to keep the media from drying out.
Scientists and technicians working in a lab have the advantage of being able to pour and proof their plates in a laminar flow hood. The average amateur brewer does not have access to a laminar flow hood. He/she has to take steps to minimize dust pickup during the proofing period. Condensation inside of a plate can be bad news in a home environment. What happens when a dish that has condensation on its lid is proofed upside down at room temperature is that house dust can settle in the gap between the lid and the bottom where it mixes with the condensation. The problem comes when the plate is turned over and the condensation drips onto the media. That's why I have found that it is best to proof a plate unsealed with the cover up in a home environment. The proofing period should be long enough to allow any condensation that formed on the lid to evaporate.
By the way, I always proof and incubate my plates with the cover up. The only time that I store my plates upside down is while they are in my brewing refrigerator (I seal my plates with Parafilm before storing them in my brewing refrigerator). Storing plates upside down in a refrigerator prevents condensation from forming in their lids. It also helps to keep the media from drying out.
Scientists and technicians working in a lab have the advantage of being able to pour and proof their plates in a laminar flow hood. The average amateur brewer does not have access to a laminar flow hood. He/she has to take steps to minimize dust pickup during the proofing period. Condensation inside of a plate can be bad news in a home environment. What happens when a dish that has condensation on its lid is proofed upside down at room temperature is that house dust can settle in the gap between the lid and the bottom where it mixes with the condensation. The problem comes when the plate is turned over and the condensation drips onto the media. That's why I have found that it is best to proof a plate unsealed with the cover up in a home environment. The proofing period should be long enough to allow any condensation that formed on the lid to evaporate.
EarlyAmateurZymurgist
Well-Known Member
- Joined
- Aug 4, 2013
- Messages
- 518
- Reaction score
- 140
It's not that I do not trust the purity of yeast producers. It's that I know that most liquid cultures are not 100% pure. Without a means by which to verify the purity of a liquid culture, one must assume that it carries a wild microbial load in addition to the domesticated yeast culture. A well-isolated colony on a plate is almost guaranteed to be 100% pure.
Here's what can happen when one inoculates slants directly from liquid cultures:
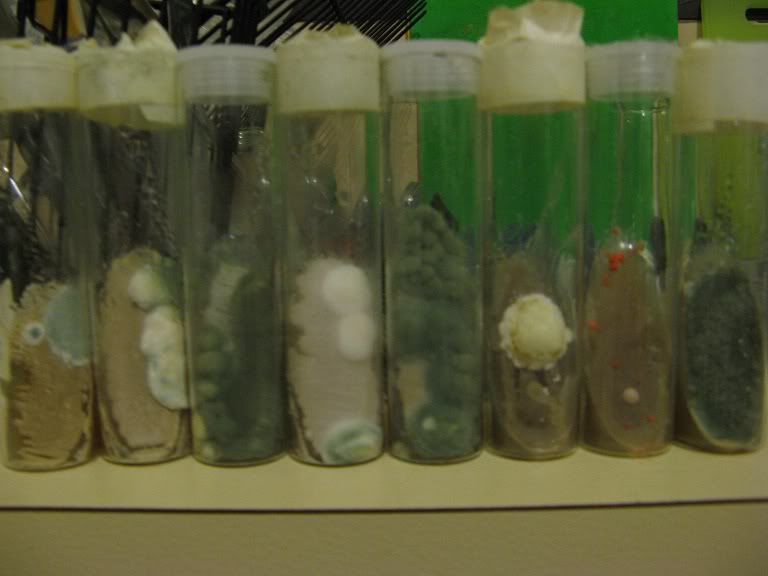
Here's what can happen when one inoculates slants directly from liquid cultures:

So this is exactly what I did with three plates. Proofed them for three days ad they looked great. Streaked them with washed Conan yeast. After two days had nice signs of growth. This was Friday and I had kept the thee plates upside down covered with al foil.
This was last Friday. Returned home from a weekend trip Sunday prepared to slant the findings only to reveal mold growing on all three plates. I had moved them from under the foil to inside an open ziplock bag
I assume this was a result of leaving them upside down. In any event I slanted five small colonies far away from the mold. I plan to do a 1 gallon batch of beer this weekend with one of the slants to proof it out.
Sent from my iPhone using Home Brew
This was last Friday. Returned home from a weekend trip Sunday prepared to slant the findings only to reveal mold growing on all three plates. I had moved them from under the foil to inside an open ziplock bag
I assume this was a result of leaving them upside down. In any event I slanted five small colonies far away from the mold. I plan to do a 1 gallon batch of beer this weekend with one of the slants to proof it out.
Sent from my iPhone using Home Brew
FredTheNuke
Well-Known Member
Now that he has panicked everyone.... That in my experience is very rare. I have almost 50 slants in my keezer for almost 24 months. About 8-10 varieties of Wyeast and White Labs liquid cultures. Nothing like the above picture has popped up. Now if I leave them at room temp for a few weeks I'm sure i'll have a few blooms of stuff I don't want. The key to them is the yeast cells that you want out-compete anything else in the wort making minor issues inconsequential.
MichaelBrock
Well-Known Member
It's not that I do not trust the purity of yeast producers. It's that I know that most liquid cultures are not 100% pure. Without a means by which to verify the purity of a liquid culture, one must assume that it carries a wild microbial load in addition to the domesticated yeast culture. A well-isolated colony on a plate is almost guaranteed to be 100% pure.
Here's what can happen when one inoculates slants directly from liquid cultures:

Have you actually found that vials of commercial yeast contain mold? I haven't had any mold in any of my direct-from-the-vial slants. If I did, I would definitely blame it on my sanitation. Never would have occurred to me that it was coming from the commercial yeast. And to be honest, I have my doubt that the mold in those slants did either.
EarlyAmateurZymurgist
Well-Known Member
- Joined
- Aug 4, 2013
- Messages
- 518
- Reaction score
- 140
Have you actually found that vials of commercial yeast contain mold?
The slants shown above are from an amateur brewer Australia who goes by the user name Wolfy. They were inoculated from commercial liquid cultures.
Trust me, commercial liquid cultures are not 100% pure. Neither are dry cultures, but at least the dry yeast manufacturers are open about it (see microbiological properties on the following data sheet: http://www.danstaryeast.com/system/files/pdfs/nottingham_datasheet_0.pdf?download=1). That level of purity is not guaranteeable at home brew trade price points. I have encountered mold and bacteria while plating several liquid cultures directly from the source. In all cases, the wild microflora was in the streak; therefore, it came from the source. I plate using aseptic technique and a nichrome loop that is red hot before being dipped into the culture; therefore, I did not drag the load into the plate. It was part of the culture.
EarlyAmateurZymurgist
Well-Known Member
- Joined
- Aug 4, 2013
- Messages
- 518
- Reaction score
- 140
So this is exactly what I did with three plates. Proofed them for three days ad they looked great. Streaked them with washed Conan yeast. After two days had nice signs of growth. This was Friday and I had kept the thee plates upside down covered with al foil.
This was last Friday. Returned home from a weekend trip Sunday prepared to slant the findings only to reveal mold growing on all three plates. I had moved them from under the foil to inside an open ziplock bag
I assume this was a result of leaving them upside down. In any event I slanted five small colonies far away from the mold. I plan to do a 1 gallon batch of beer this weekend with one of the slants to proof it out.
If the mold is in the streak path, it was transferred to the plate with the culture; therefore, the plate did its job. Plating is how we separate yeast from mold and other wild microflora. If the mold is in random places on your plates, then you need to revisit your process.
EarlyAmateurZymurgist
Well-Known Member
- Joined
- Aug 4, 2013
- Messages
- 518
- Reaction score
- 140
The key to them is the yeast cells that you want out-compete anything else in the wort making minor issues inconsequential.
Most infections occur at an early stage during propagation. Every propagation step is an opportunity for an infection to multiply. Bacteria cells divide every thirty minutes whereas yeast cells divide every ninety minutes. Under the right conditions, bacteria cells can divide every twenty minutes, which translates to 2^18 = 262,144 bacteria cell divisions in six hours versus 2^4 = 16 yeast cell divisions. That's why it is important to bank a wild microflora-free culture.
The number one comment that I get when someone sips one of my beers is "Wow, that's really clean and smooth. How do you avoid off-flavors?" I do not ferment ales at low temperatures. In fact, I do not use temperature control. I brew with the seasons. I pitch very clean cultures that do not see non-autoclaved media until they are stepped to 1L.
With that said, if good aseptic transfer technique is maintained, a culture only needs to be plated for singles one time. In my humble opinion, the extra step is more than justified by having banked cultures that will remain clean for years if not decades.
If one does not want to go through the hassle of making plates, disposable pre-poured malt agar plates can be purchased for around $2.00 to $3.00 each in packs of 10. A quality borosilicate glass petri dish costs more than $3.00. I am seriously considering switching to pre-poured plates after years of making my own plates.
FredTheNuke
Well-Known Member
I may play with plates once I get my dedicated brewery finally up and running. Maybe add a small dorm fridge to keep just my yeast bank in at 34 degrees (or as damn close as I can get).
I haven't had any problems with mold or other infections when using commercial liquid yeast to plate or slant. I work near a flame right now but do plan to build a flow hood soon. If you love the intricacies of brewing then a yeast bank is for you. A big thanks to the original poster for a clear and accurate tutorial. Most of the way I keep and propagate my yeast came from this thread. I have read a lot on the subject and this is a straight forward and easy to follow method. Thanks again.
EAZy,
Thanks for the info. I purchased some Pyrex plates so I can begin plating at home per your suggestions. Question tho, if you say the liquid cultures are not 100% pure, if we do plate and isolate single colonies, how do we differentiate between desired yeast single colonies and wild yeast single colonies?
It may not be ideal, but I have been culturing from vials and packs to proofed slants without any problems for over a year now. I do have a good amount of experience in labs from HS and college though I don't work in one, so I feel I have some little advantage over most looking at doing this.
Thanks
Sent from my Nexus 7 using Home Brew mobile app
Thanks for the info. I purchased some Pyrex plates so I can begin plating at home per your suggestions. Question tho, if you say the liquid cultures are not 100% pure, if we do plate and isolate single colonies, how do we differentiate between desired yeast single colonies and wild yeast single colonies?
It may not be ideal, but I have been culturing from vials and packs to proofed slants without any problems for over a year now. I do have a good amount of experience in labs from HS and college though I don't work in one, so I feel I have some little advantage over most looking at doing this.
Thanks
Sent from my Nexus 7 using Home Brew mobile app
HollisBT
Well-Known Member
Question for those who slant yeast:
When you are propagating a culture to pitch to a beer, how do you estimate your starting cell count? Ie: when you are calculating your starters and how many cells your beer will need, what number do you start with in the calculator? 1 million? More? Less? RDWHAHB?
When you are propagating a culture to pitch to a beer, how do you estimate your starting cell count? Ie: when you are calculating your starters and how many cells your beer will need, what number do you start with in the calculator? 1 million? More? Less? RDWHAHB?
MichaelBrock
Well-Known Member
Question for those who slant yeast:
When you are propagating a culture to pitch to a beer, how do you estimate your starting cell count? Ie: when you are calculating your starters and how many cells your beer will need, what number do you start with in the calculator? 1 million? More? Less? RDWHAHB?
I figure that the yeast in a slant added to 10ml of wort and fermented out will have .8 billion cells. I posted about that, including where I got that information, earlier in this thread:
https://www.homebrewtalk.com/f163/slanting-yeast-133103/index59.html#post5779675
tally350z
Well-Known Member
Does anyone take a loop from the wyeast smack packs? I will be getting the three private collect yeast starins 1203, 2487, 3864, and would like to streak a plate with each. Does anyone have any tips or should I just take the loop after I pour the yeast out into my starter? What about make a sterile wort in a 50ml beaker and than dumping some of the smack pack into that, like a small starter and than using that to plate from?
I'm not sure that this has been mentioned yet, but it appears cynmar sells malt extract prepared plates already.... $11/10 plates... doesn't seem like a bad deal even if it costs $20 to ship 2 day/overnight (not sure what shipping actually costs). Has anyone tried these vs making your own?
Does anyone take a loop from the wyeast smack packs? I will be getting the three private collect yeast starins 1203, 2487, 3864, and would like to streak a plate with each. Does anyone have any tips or should I just take the loop after I pour the yeast out into my starter? What about make a sterile wort in a 50ml beaker and than dumping some of the smack pack into that, like a small starter and than using that to plate from?
I usually sanitize the outside of the pack, shake it up, open with sanitized scissors, pour contents into my starter, then use a flamed loop to transfer directly onto my proofed slants. I've done this with multiple strains without a problem.
Sent from my Nexus 7 using Home Brew mobile app
tally350z
Well-Known Member
I am not sure what I am doing wrong with making the media. I measure the amounts and put in a flask to boil for a minute. Than I pour into a beaker and autoclave the media for 20 minutes. After I pull out the beakers and vials, each one has clumps in the bottom. It never looked like that when I added the media before autoclaving it.
I'm sure you'll get more experienced replies.
But, what has worked for me was starting with warm water in a pot, and frequent stirring with a whisk (not so vigorous as to aerate) while it heats up to a simmer. I never brought it to a boil before going into the pressure canner.
But, what has worked for me was starting with warm water in a pot, and frequent stirring with a whisk (not so vigorous as to aerate) while it heats up to a simmer. I never brought it to a boil before going into the pressure canner.
i have moisture in my slants. the medium dried solid but each slant had a little moisture on top after drying and then when I stand the slant up the moisture runs under the slanted medium and the medium spins around in the slant.
will this be a problem? I've tried making slants twice and it happened both times. I boil the dme and agar before adding to th slant for 15 minutes so everything is disolved and mixed together well. it seems the moisture is from the steam from the pressure cooker and it doesn't mix in with the medium wbile drying. I cant find any information about this.
me recipe is:
300mL of water
4 grams of agar
22 grams of DME
2 grams of nutrient
will this be a problem? I've tried making slants twice and it happened both times. I boil the dme and agar before adding to th slant for 15 minutes so everything is disolved and mixed together well. it seems the moisture is from the steam from the pressure cooker and it doesn't mix in with the medium wbile drying. I cant find any information about this.
me recipe is:
300mL of water
4 grams of agar
22 grams of DME
2 grams of nutrient
MichaelBrock
Well-Known Member
I am not sure what I am doing wrong with making the media. I measure the amounts and put in a flask to boil for a minute. Than I pour into a beaker and autoclave the media for 20 minutes. After I pull out the beakers and vials, each one has clumps in the bottom. It never looked like that when I added the media before autoclaving it.
I get this too. I thought it was undissolved yeast nutrient but others here think that it is hot break. Either way it hasn't been an issue for me and I doubt it will be for you.
MichaelBrock
Well-Known Member
i have moisture in my slants. the medium dried solid but each slant had a little moisture on top after drying and then when I stand the slant up the moisture runs under the slanted medium and the medium spins around in the slant.
will this be a problem? I've tried making slants twice and it happened both times. I boil the dme and agar before adding to th slant for 15 minutes so everything is disolved and mixed together well. it seems the moisture is from the steam from the pressure cooker and it doesn't mix in with the medium wbile drying. I cant find any information about this.
me recipe is:
300mL of water
4 grams of agar
22 grams of DME
2 grams of nutrient
I had this same issue. In addition, yeast would get in the liquid and make its way under the agar. It would grow, produce CO2 and push the plug of agar right out of the tube when I opened it. I tried leaving the vials cracked open a bit for 5 or so days when first making them to dry out and that helped. Ultimately, I started making the slants with a steep enough angle that the agar does not completely cover the bottom of the vial. That keeps the liquid & yeast from getting under the agar. And for some reason my last batch of slants doesn't have the moisture my previous batches did...no idea why.
Similar threads
- Replies
- 8
- Views
- 3K
- Replies
- 3
- Views
- 742
- Replies
- 17
- Views
- 2K









































