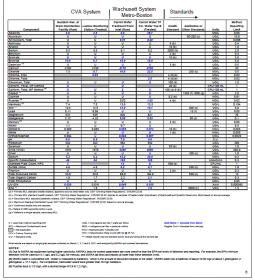
I am trying to determine my RA for use in palmer excel spreadshhet. Can anyone help with the math. Below is my water data.
Alkalinity 4.0
Calcium 2.15
Chloride 8.7
Hardness 7.5
Magnesium 0.52
pH 6.9
Potassium 0.481
Sodium 5.5
Sulfate (SO4) 4.5
Alkalinity 4.0
Calcium 2.15
Chloride 8.7
Hardness 7.5
Magnesium 0.52
pH 6.9
Potassium 0.481
Sodium 5.5
Sulfate (SO4) 4.5