Cybershadow
Well-Known Member
Mash ph seems a little high, what do you guys think? Not sure of the best way to adjust lower.






Yes, I would say it's a bit high. Calcium is used to lower PH, but the calculations can be pretty complex.
Specialty grains also will lower it. Base malts typically raise the pH, where choc, roasted, caramel etc. lower it.
Easiest way would be to get some 5.2 stabilizer. Can't really go wrong with that. Five star makes one.
Try acid malt in your grain bill. Here's a comment from PassedPawn when I had a question about aciduated malt.
"For each 1% of your grain bill that is acidulated malt, you'll lower the mash pH 0.1%."
So, if your expected mash pH is 0.3% higher than you want it, try subbing 3% of your grist for aciduated malt.






![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)





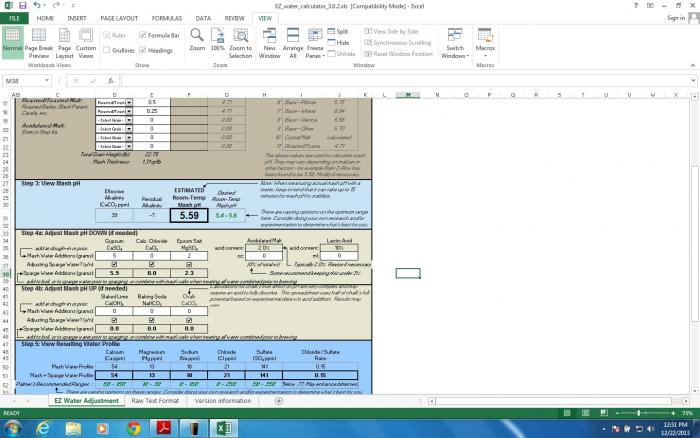
Hm, this seems a little low to me... but I've never adjusted my mash pH so I'm not exactly sure how much higher 5.59 really is than 5.2
Let's try an example for the OPs scenario. To lower his mash to 5.2 from 5.59, he would need almost 70% of his grain bill to be acidulated malt.
%acidulated = (estimated mash pH - desired mash pH)/(estimated mash pH*0.001)
(5.59-5.2)/(5.59*0.001)=69.8% acidulated malt
The numerator is the amount that the pH must be dropped, and the denominator is the amount the pH is dropped per % acidulated malt added to grain bill. The 0.001 represents the 0.1% reduction in pH. We can see how crazy this is a little easier if you look at it this way: substituting 1% acidulated malt would decrease the pH by 5.59*0.001=0.00559... and the pH has to be reduced by 0.39! That's a lot of acidulated malt.
Someone please correct me if I'm wrong, but it looks like for a difference in pH of what the OP has, this just isn't an option. Either that, or the numbers are off? (Maybe not 0.1% reduction in pH, but 0.1 reduction? or 10% reduction?).
Can someone find my error or clarify? haha
Easiest way would be to get some 5.2 stabilizer. Can't really go wrong with that. Five star makes one.
afr0byte said:Yes, it's .1 pH reduction..not %
All good advice, I am assuming I can add the acid to the mash after I take a ph reading?
Hm... didn't realize 5.2 stabilizer was that frowned upon. A jar of it came from the guy I bought my equipment from, and I've been using it since going all-grain. pH has been right at 5.2 every time... maybe I'm just lucky and have good water then... Thanks for the advice though!
I'm just measuring with pH strips (4.6-6.2 range). So I know it's not the most accurate thing in the world, but it shows pretty dang close.
I'm going to brew a batch this weekend. I'll leave out the stabilizer and let you know what I get.
So what does 5.2 stabilizer typically do? Lower the pH, right?
Interesting. Thanks Yooper.
I've only done 2 all-grain batches, and the first one I had some trouble with. I'm going to do a couple more as-is to get an idea of what my efficiency is (first batch was all-over the place, so I didn't bother. Missed mash temp and boil volume pretty bad. Second batch was around 68% i think)
Next step after that will be sending out a sample to Ward and getting into water chemistry. So I'm not really there yet. But I will get a pH reading when I start the mash, then I'll add it and see how it affects my pH.