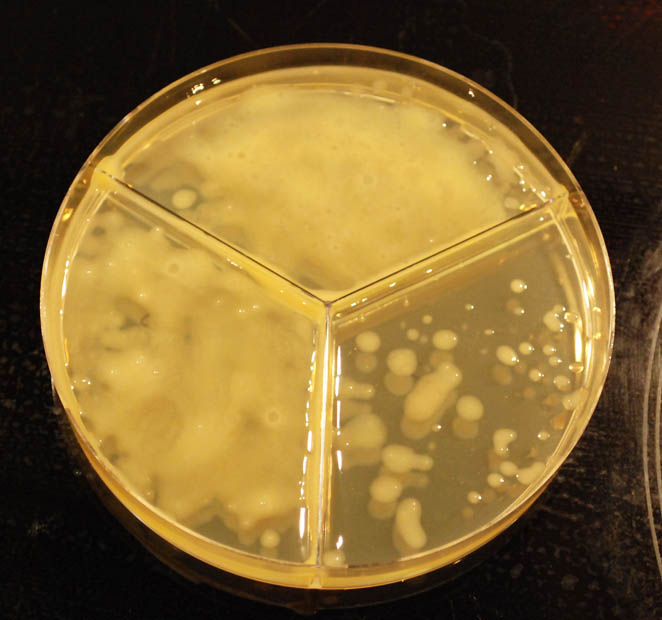
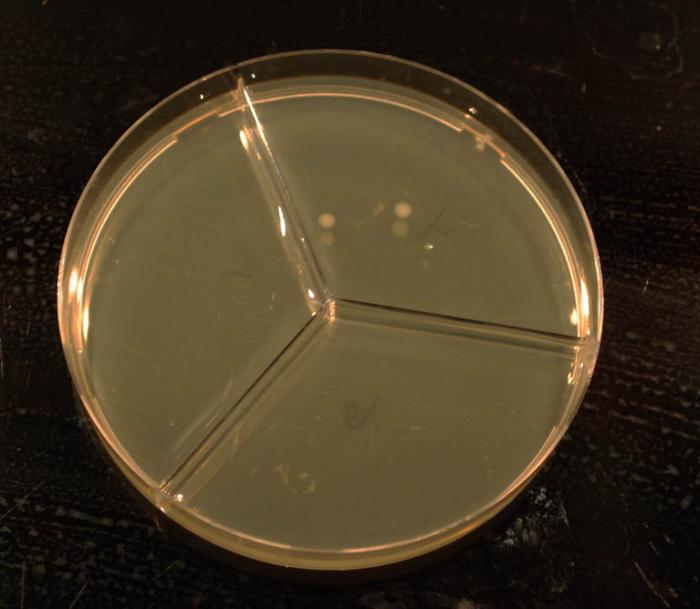
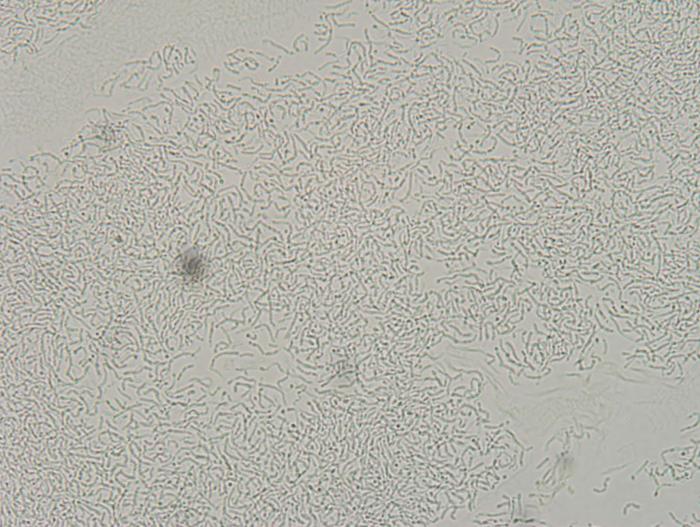
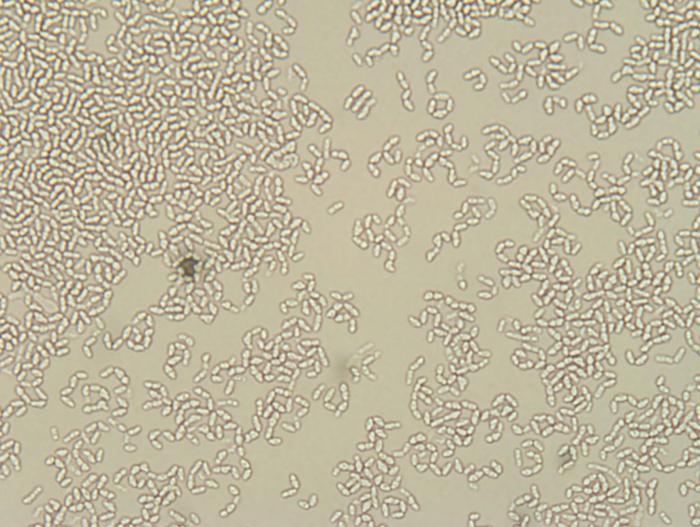
dinnerstick
Well-Known Member
from the PLoS paper that someone just posted in this very forum
http://www.plosone.org/article/info:doi/10.1371/journal.pone.0035507
For amplification of the ITS1/ITS4 domain of yeast 26S rDNA genes (ITS-TRFLP) [5], the forward primer used was ITS1HEX (5′-[5HEX] TCCGTAGGTGAACCTGCGG-3′ and the reverse primer was ITS4 (5′-TCCTCCGCTTATTGATATGC-3′
and the reverse primer was ITS4 (5′-TCCTCCGCTTATTGATATGC-3′ [6]. The PCR conditions were an initial denaturation at 95°C for 2 min, followed by 30 cycles of denaturation at 95°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 2 min, and with a final extension at 72°C for 7 min.
[6]. The PCR conditions were an initial denaturation at 95°C for 2 min, followed by 30 cycles of denaturation at 95°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 2 min, and with a final extension at 72°C for 7 min.
not even degenerate primers, so i guess they anneal to sacc, brett, candida, etc? haven't read the paper yet though, just the abstract and tiny bit of methods. tired. tomorrow.
http://www.plosone.org/article/info:doi/10.1371/journal.pone.0035507
For amplification of the ITS1/ITS4 domain of yeast 26S rDNA genes (ITS-TRFLP) [5], the forward primer used was ITS1HEX (5′-[5HEX] TCCGTAGGTGAACCTGCGG-3′
not even degenerate primers, so i guess they anneal to sacc, brett, candida, etc? haven't read the paper yet though, just the abstract and tiny bit of methods. tired. tomorrow.





























![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)