Northern_Brewer
British - apparently some US company stole my name
Whats your recipe for the sugar solution?
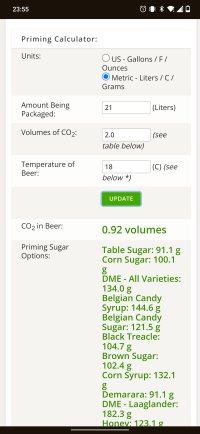
Yeah - that. In theory you can get almost 2 weights of sucrose into 1 weight of water, but 1:1 is convenient. Growing up on cask beer and generally making golden ales, I don't like my bottles to be too fizzy, so I'm typically adding about 4g/l of white sugar, so 2ml of a 1:1 solution to a 500ml bottle and about 1.7ml for a 330ml bottle (they need a bit more, relatively).Usually I'm mixing almost equal amounts by weight of water and sugar.
Willamette and Cascade wouldn't normally be first choices for dry hopping in any case, as dry hopping is all about the volatile aroma compounds that would be driven off by adding at higher temperatures, which those two don't have so much of. And in any case they would likely be swamped by the Galaxy you'd added previously.I used galaxy hops during the process but I've got maybe 100 grams each of Willamette and cascade and I'm considering putting a 2 gallon into a 2 carboys and dry hopping to see what it comes out like.
I'd end up with 3 gallons of the original, 1 gallon of dry hopped Willamette and 1 gallon of dry hopped cascade. Do you think it's worth doing?
So you can do it, but I wouldn't if it was me, just KISS and resist the temptation to fiddle at this stage.



















![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)






































