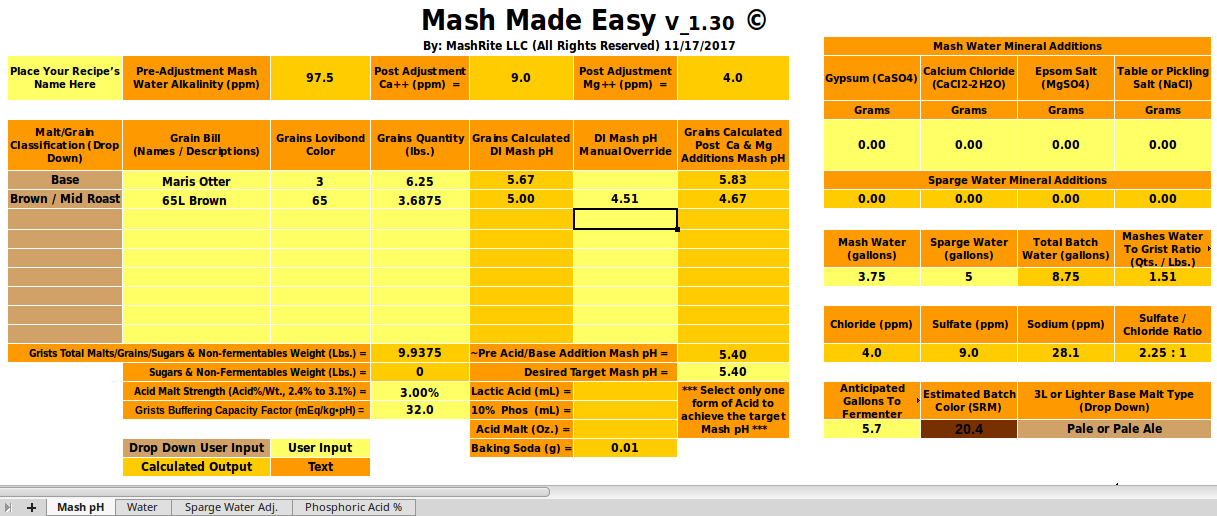
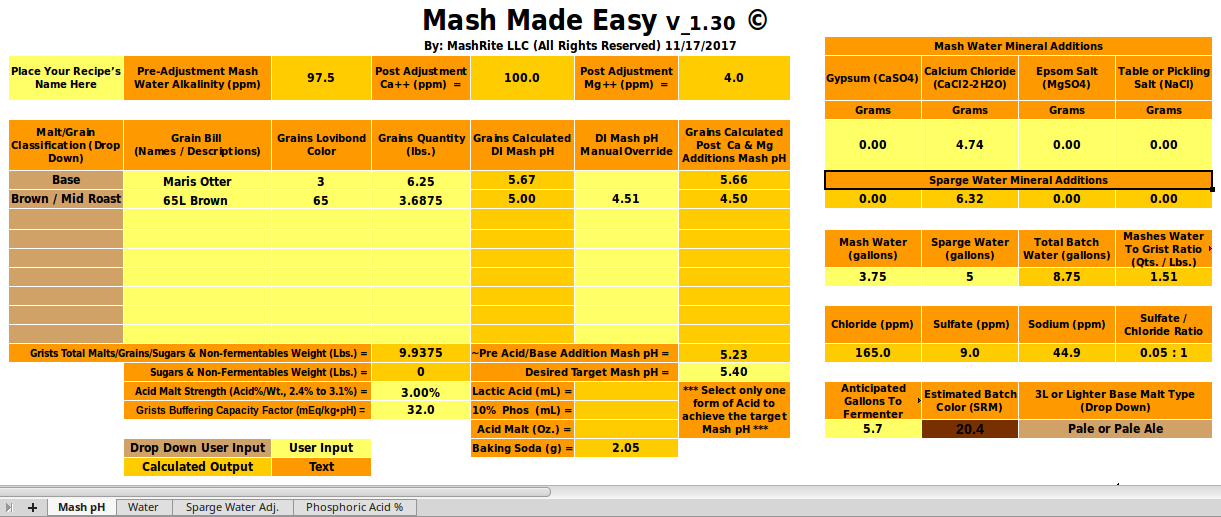
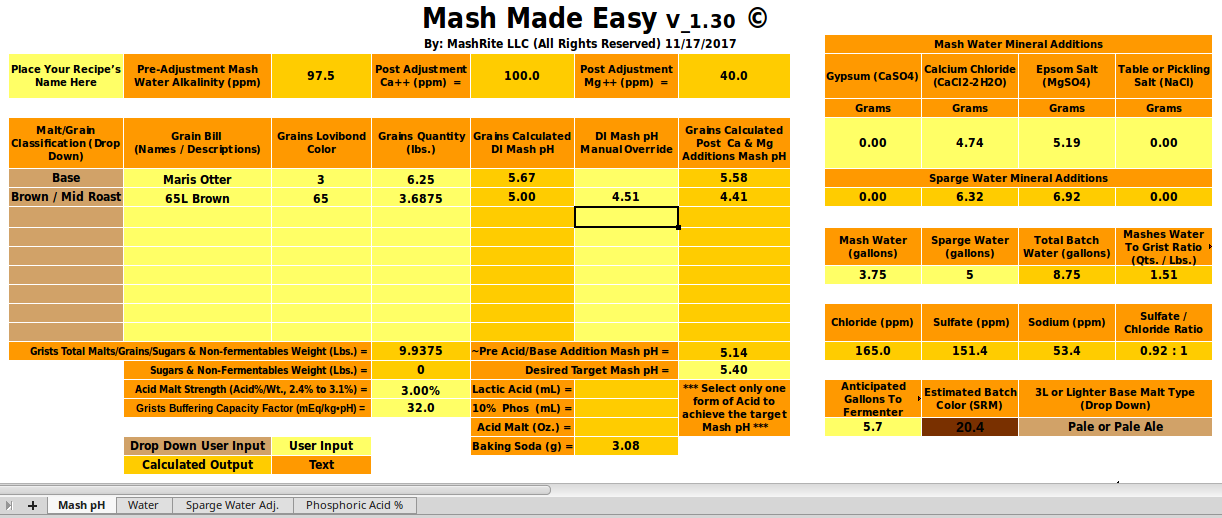
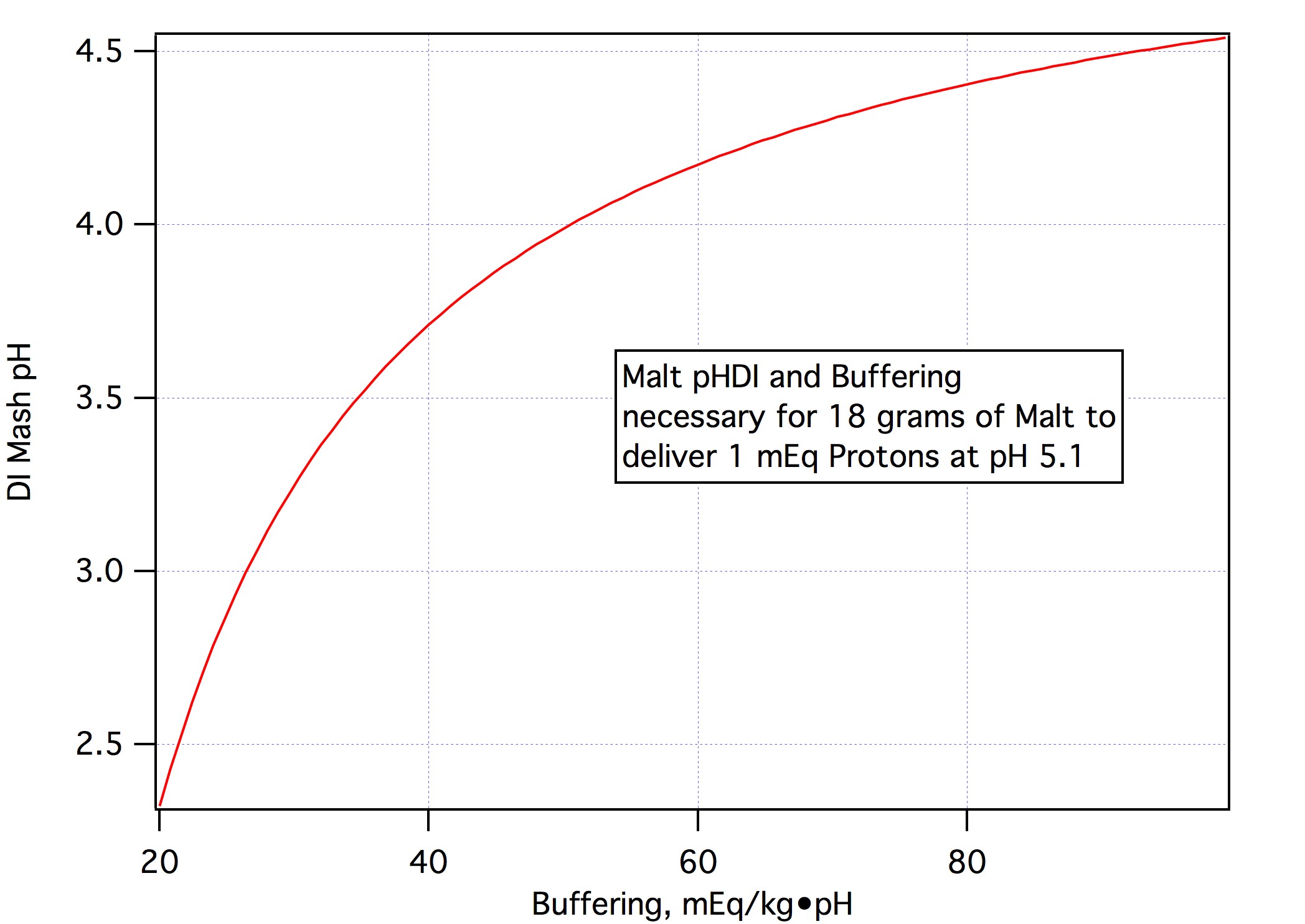
Well, I'm not used to micro-scale testing,...[/QUOTE[ Think of this as a check rather than a formal test. Were we to want a firm number for a we would take three or more 100g samples of this malt, add 0, 1, 2, 3, 4... mmol NaHCO3, to 100 100 mL DI water, put the samples in a water bath, add the water to the samples at two minute intervals and then, at 20 minutes measure the first sample's pH, at 22 measure the second and so on. This would give us a curve of mEq/kg vs pH and we would then fit a line (or parabola if it seemed justified) through the data to get our estimate of a. Note that I am not asking that you do this. You have shown that the buffering of this malt is much higher than normal and until someone wants to go out and do a fuller set of measurements that's going to have to suffice. I, for one, appreciate that you were willing to take the time to do this measurement and hope the other participants here do too.













































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)