I have been quietly crunching these water compositions. Time to start getting noisy again.
I'm finding the results (so far) very revealing. I have for years (since pre-Covid times) been mystified by low pH levels in my mashes (lower than pH 5.0) and getting nowhere trying to explain it ... or finding others on various forums to explain it for me. But these "tests" over the past couple of days have been turning up plenty of explanations and corrections.
They are tests, performed on a calculator (Bru'n Water), and will need confirmation from real brew trials. I'm only outlining what I'm up to here on the chance that someone has already done the work (so I won't have to!). There are no hundredths of a gram! Although I did use "hundredths of a gram of get the rounding going the right way.
I already brew "full-boil-length-mashes". Using "Grainfather" setups ("full metal jacket" BIAB ... with recirculation). My 70L 3V System has been out-of-commission for over a year. All these recent tests have been "full-boil-length-mashes". Mashing like that appears to have exacerbated my problems so I couldn't reach a swift resolution.
The following tables are rough, unfinished, and need a bit more smoothing out. Using what @Silver_Is_Money refers to as "quasi-empirical" techniques; I had to look that up:
quasi-
/ˈkweɪzʌɪ,ˈkweɪsʌɪ,ˈkwɑːzi/
combining form
Oh wow! Got to have some of that (it's right up my street!).
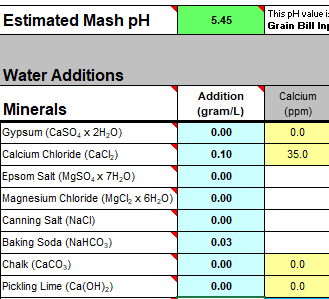
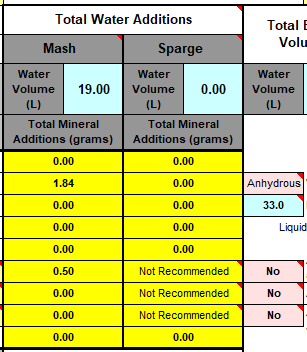
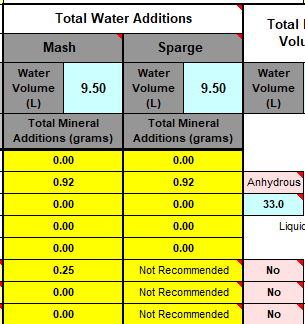
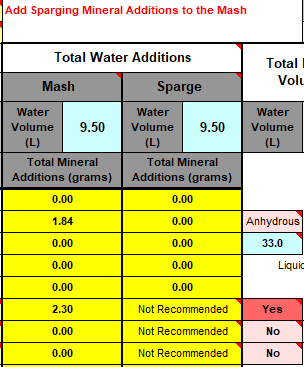
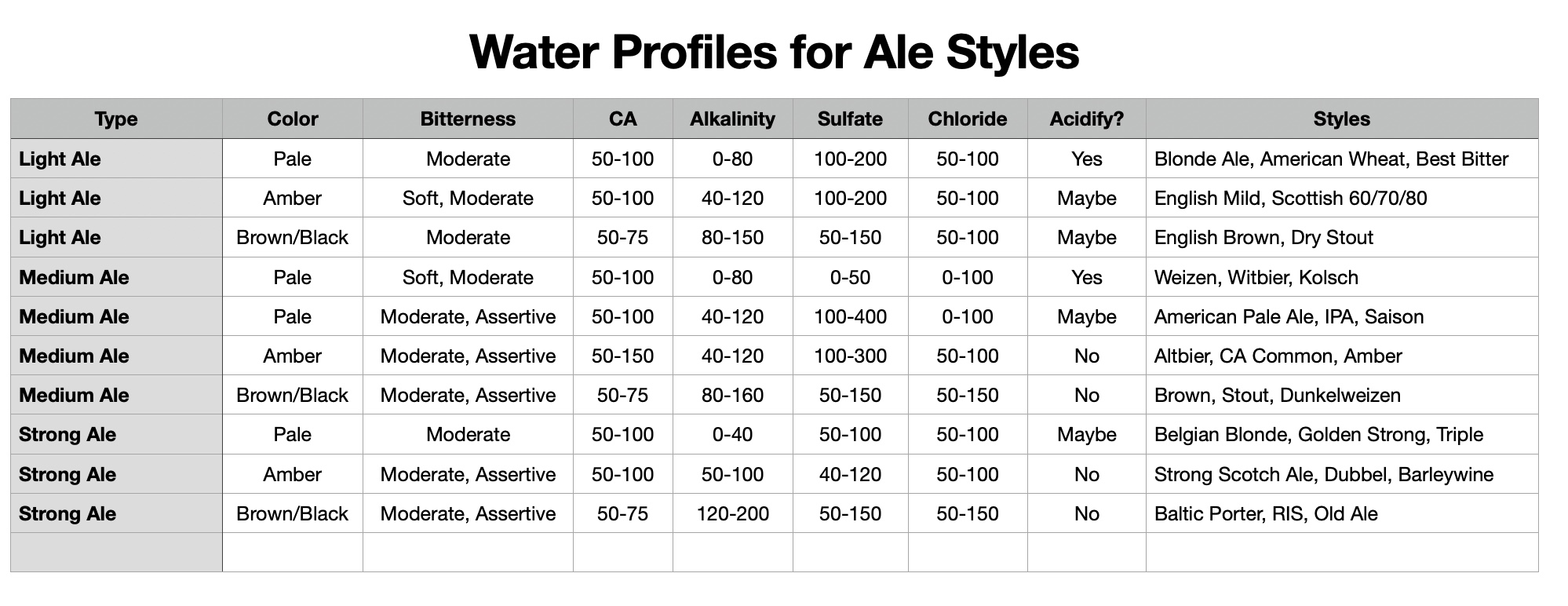
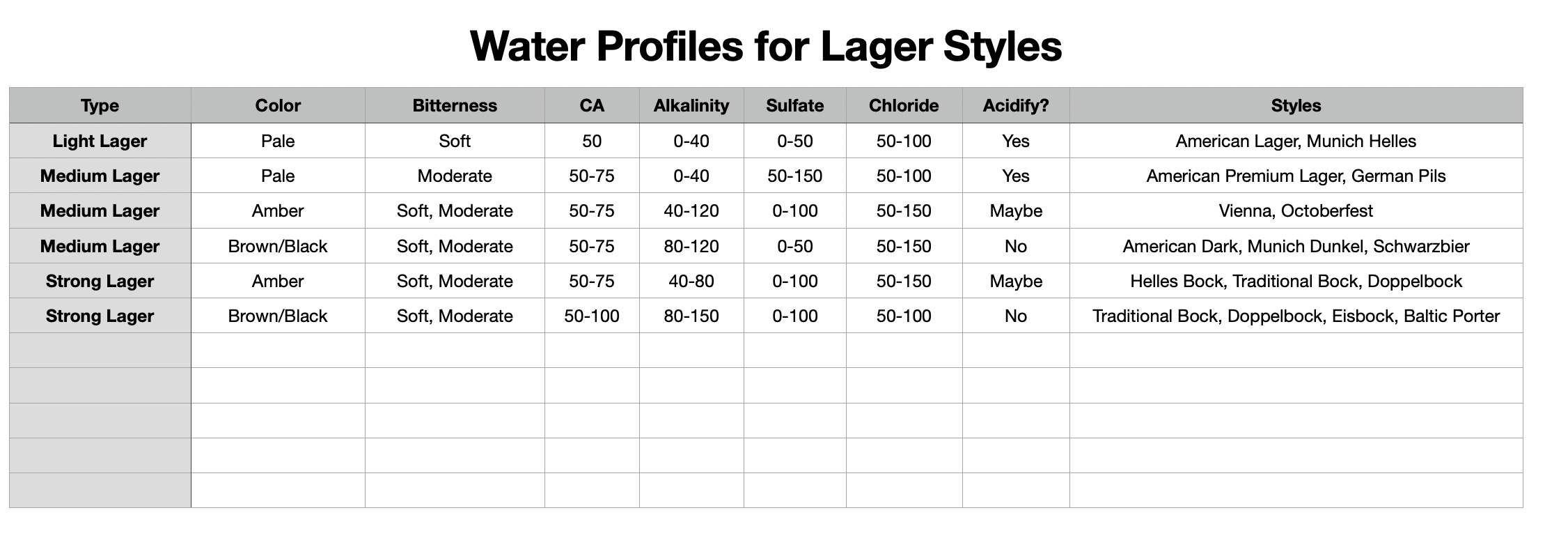
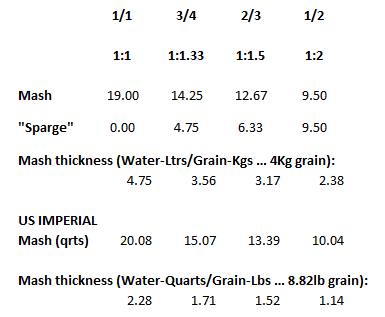
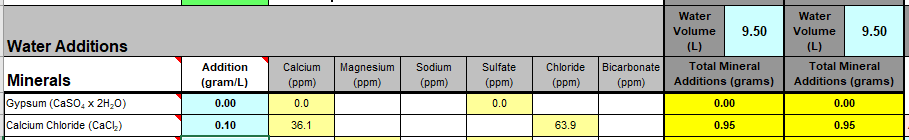
I'll need to fill in some more scenarios too. Then crunch the whole lot down into a simple tool (Spreadsheet). Meanwhile, what I'm doing is stripping some, what I consider, are un-necessary variables, i.e. most of the salt additions in the mash, and the free-for-all mash-thicknesses to just two? Maybe an in-between third? Calcium additions to be fixed, I'm counting on 35ppm Ca being sufficient for the mash (as CaCl2). Alkalinity remains a variable, but its limited scope in these situations ought to make it easier to handle? All other additions for the boil where their influences, not being mash specific, wont impact the mash (like the effect of Ca and Mg on mash acidity, the source of many of my current issues.).
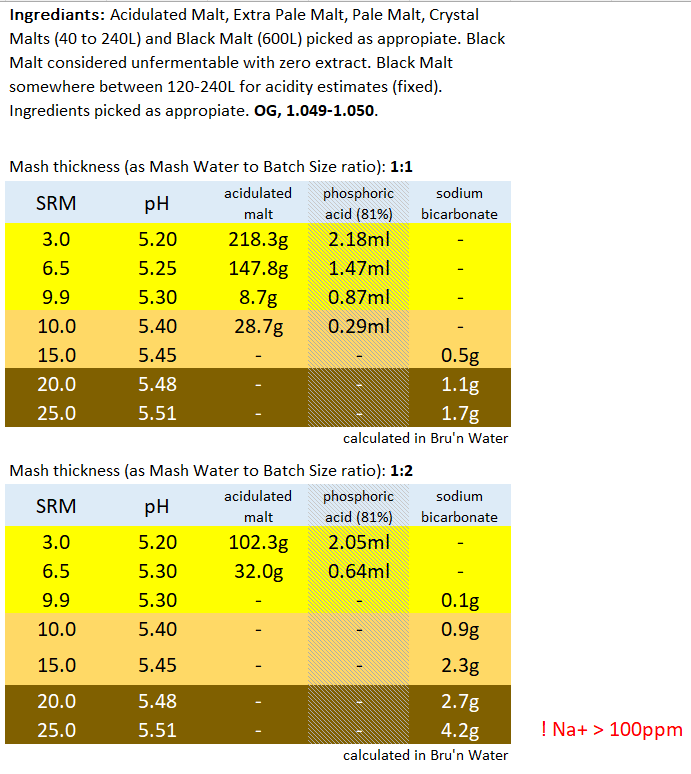
This is the table extracted (full boil length mash) so far:
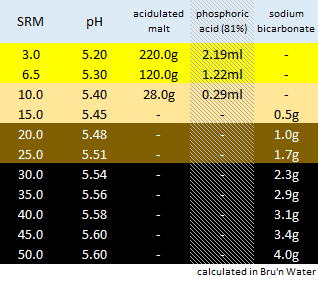
Divided Yellow, Amber, Brown and Black. Goes to 50SRM black, which is the limit for Bru'n Water. Alternative for somewhat unpredictable Acidulated Malt is provided wits Phosphoric Acid (81%). The pH predictions are what I'm mainly stealing from Bru'n Water, but I do appreciate what a notoriously tricky task it is pairing pH with a handful of salts.
More than enough fo now ... I'm off to bed! The question is ... there will be those who've trodden this path before ... I hope to learn the pitfalls before embarking on this much further. I'll attempt to explain more (if neded) tomorrow?
Thanks.
I'm finding the results (so far) very revealing. I have for years (since pre-Covid times) been mystified by low pH levels in my mashes (lower than pH 5.0) and getting nowhere trying to explain it ... or finding others on various forums to explain it for me. But these "tests" over the past couple of days have been turning up plenty of explanations and corrections.
They are tests, performed on a calculator (Bru'n Water), and will need confirmation from real brew trials. I'm only outlining what I'm up to here on the chance that someone has already done the work (so I won't have to!). There are no hundredths of a gram! Although I did use "hundredths of a gram of get the rounding going the right way.
I already brew "full-boil-length-mashes". Using "Grainfather" setups ("full metal jacket" BIAB ... with recirculation). My 70L 3V System has been out-of-commission for over a year. All these recent tests have been "full-boil-length-mashes". Mashing like that appears to have exacerbated my problems so I couldn't reach a swift resolution.
The following tables are rough, unfinished, and need a bit more smoothing out. Using what @Silver_Is_Money refers to as "quasi-empirical" techniques; I had to look that up:
quasi-
/ˈkweɪzʌɪ,ˈkweɪsʌɪ,ˈkwɑːzi/
combining form
- apparently but not really
Oh wow! Got to have some of that (it's right up my street!).
I'll need to fill in some more scenarios too. Then crunch the whole lot down into a simple tool (Spreadsheet). Meanwhile, what I'm doing is stripping some, what I consider, are un-necessary variables, i.e. most of the salt additions in the mash, and the free-for-all mash-thicknesses to just two? Maybe an in-between third? Calcium additions to be fixed, I'm counting on 35ppm Ca being sufficient for the mash (as CaCl2). Alkalinity remains a variable, but its limited scope in these situations ought to make it easier to handle? All other additions for the boil where their influences, not being mash specific, wont impact the mash (like the effect of Ca and Mg on mash acidity, the source of many of my current issues.).
This is the table extracted (full boil length mash) so far:

Divided Yellow, Amber, Brown and Black. Goes to 50SRM black, which is the limit for Bru'n Water. Alternative for somewhat unpredictable Acidulated Malt is provided wits Phosphoric Acid (81%). The pH predictions are what I'm mainly stealing from Bru'n Water, but I do appreciate what a notoriously tricky task it is pairing pH with a handful of salts.
More than enough fo now ... I'm off to bed! The question is ... there will be those who've trodden this path before ... I hope to learn the pitfalls before embarking on this much further. I'll attempt to explain more (if neded) tomorrow?
Thanks.



 ).
).


























![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)