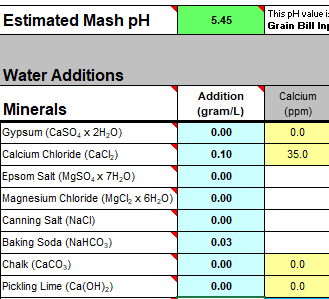
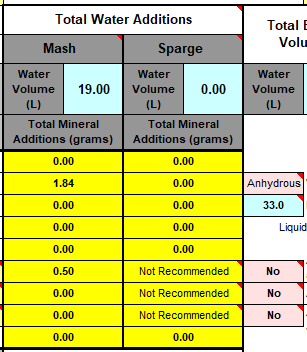
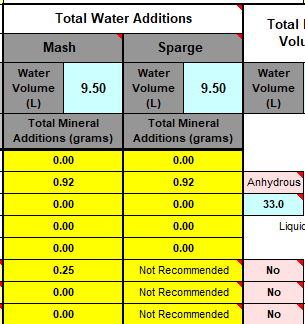
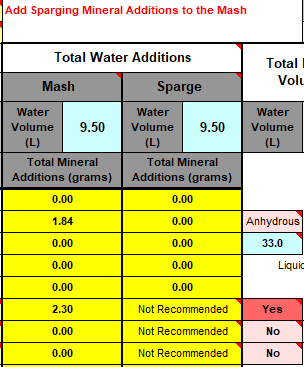
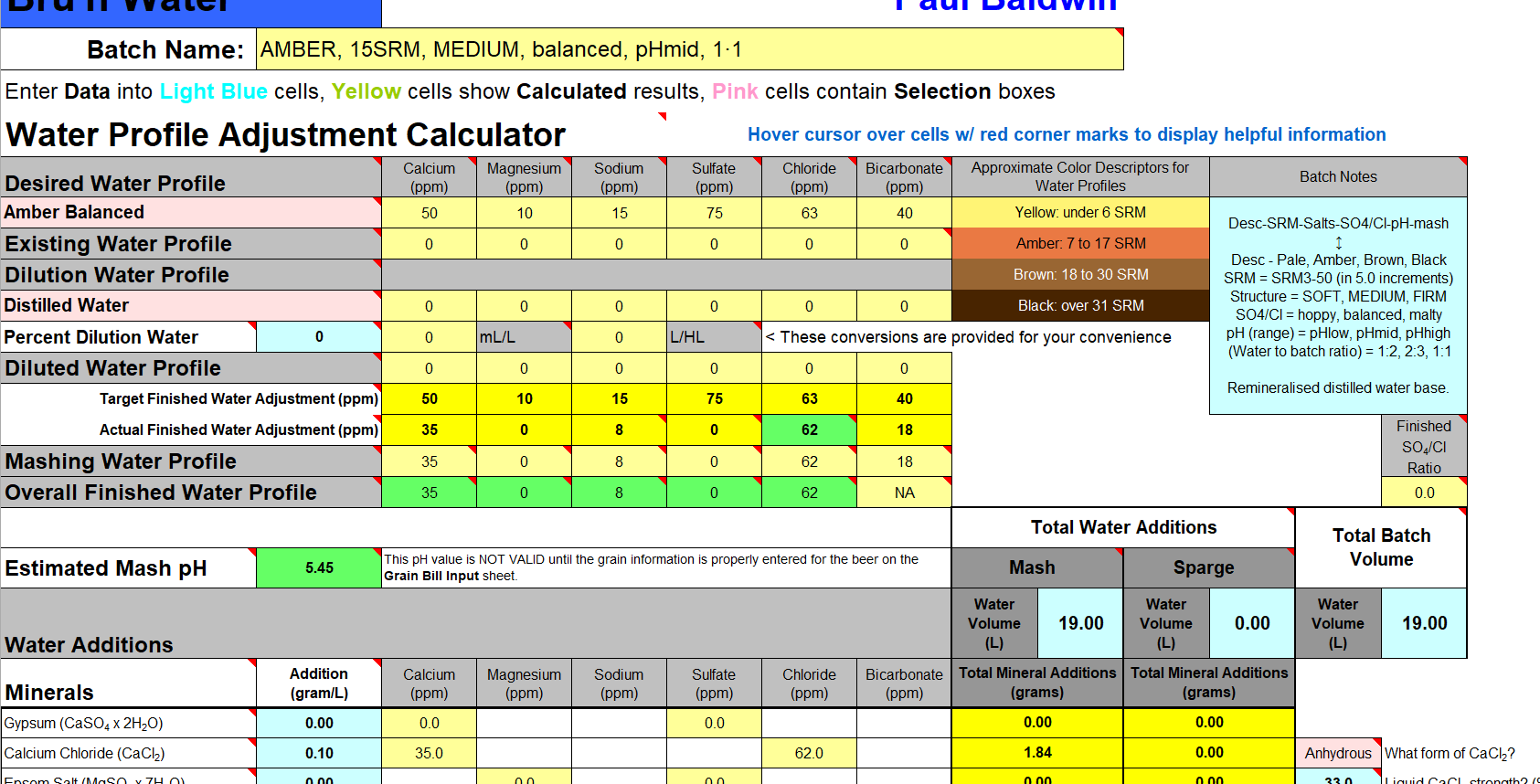
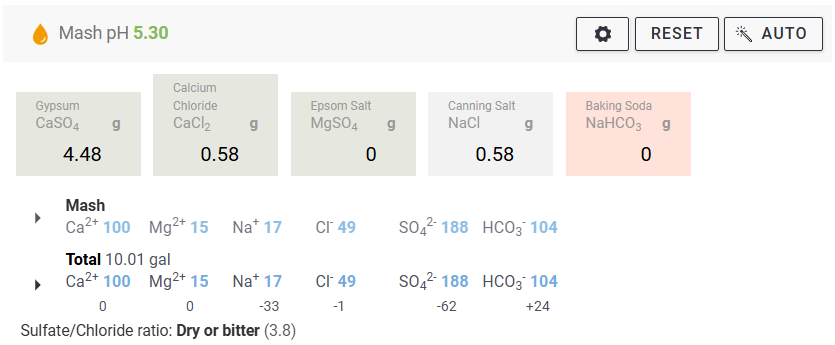
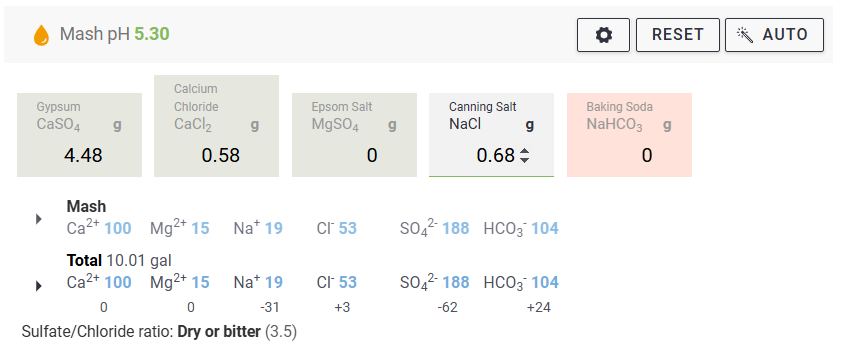
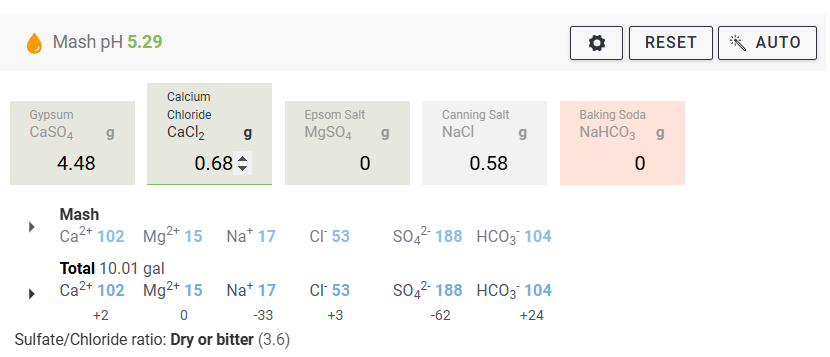
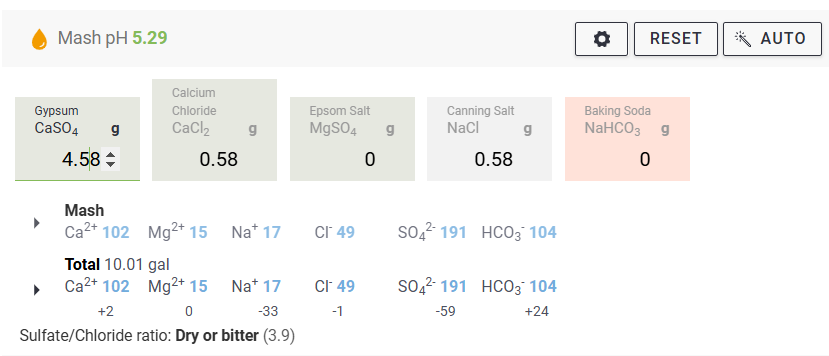
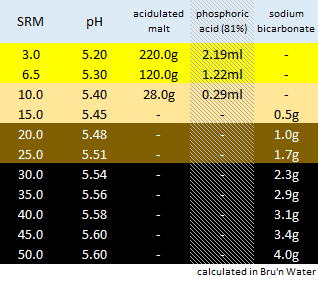
We measure our water salts to what we think is one hundredth of a gram (two decimal places of a gram). If we do that accurately we must be getting the water additions as originally intended for the style of beer being brewed. Should we not?
Yes, you should ... If your mash to water ratio is identical to the original's. If, your sparge water (and any top-up water) is treated to be identical to the original's. If, you collect identical proportions of wort as the original. If, your "boil-off" is identical to the original. If, ... is there more?
Otherwise, your "accurate" weighing out water salts is meaningless? Even tenth of a gram, or within a gram, makes little sense.
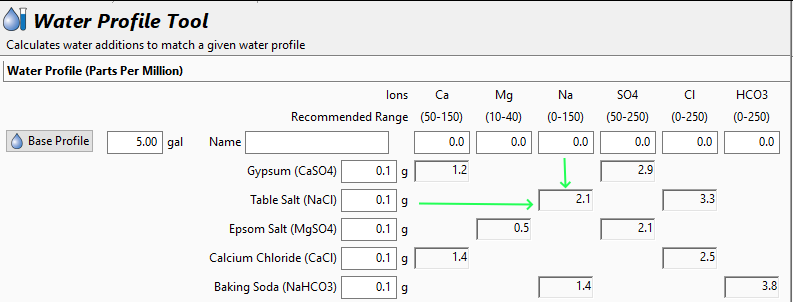
Water chemicals are described as a concentration (grams per litre, etc.). Chemical reactions are described as an amount reacting with a specific amount. Flavours (with flavouring enhancers, such as already in the water) will depend on concentrations. Malt, sugars, hops, etc., (the source of reactants and flavours) will also be described as concentrations ... but in entirely different "units" (pounds per gallon, kilos per "batch", etc.) and those units not converted.
Why are so many of us obsessed with weighing things (like water salts) out to miniscule proportions? And other directly related things not weighed or measured to the same degree of accuracy?
Yes, you should ... If your mash to water ratio is identical to the original's. If, your sparge water (and any top-up water) is treated to be identical to the original's. If, you collect identical proportions of wort as the original. If, your "boil-off" is identical to the original. If, ... is there more?
Otherwise, your "accurate" weighing out water salts is meaningless? Even tenth of a gram, or within a gram, makes little sense.
Water chemicals are described as a concentration (grams per litre, etc.). Chemical reactions are described as an amount reacting with a specific amount. Flavours (with flavouring enhancers, such as already in the water) will depend on concentrations. Malt, sugars, hops, etc., (the source of reactants and flavours) will also be described as concentrations ... but in entirely different "units" (pounds per gallon, kilos per "batch", etc.) and those units not converted.
Why are so many of us obsessed with weighing things (like water salts) out to miniscule proportions? And other directly related things not weighed or measured to the same degree of accuracy?






















![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)





































 ).
).