Been doing research into water for brewing . I read the water primer section. Have my water acids and minerals . I finally got my water test back.
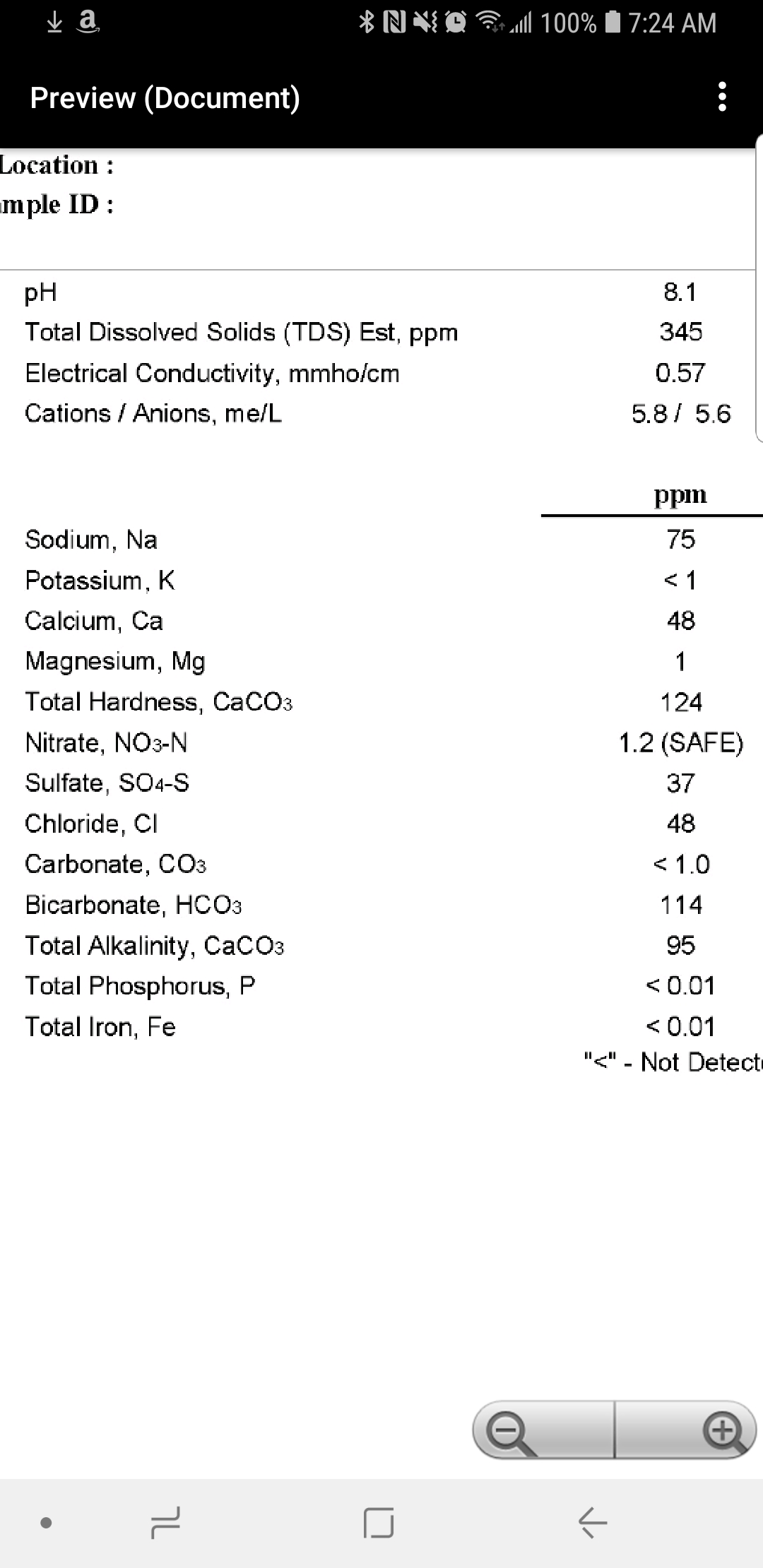



I would just add acid. For an NEIPA I would also add some calcium chloride. Your sulfate level may be too high for NEIPA's though. You will just have to try it and find out. Or at least that's what I would do.
Thanks Silver . I should be able to input it on Brun water and play with the numbers to get it close . Wonder if cutting it with RO water would help with the Neipa.











If you're going to do that, you might as well use all RO and just add minerals to it.
If I add acid to my mash water before dough in and get down to say 5.4 . When I add base malts will the ph drop below the desired ph and then I would have to add something to bring it up?
Alkalinity is the amount of acid you must add to a water (or malt) sample to get it to reach a standard pH level (usually 4.5 in the case of water). Thus you need a pH meter (or other means of determining pH such as a colored indicator dye) and a means of dispensing acid in accurately metered amounts in order to measure alkalinity.Alkalinity is checked with a ph meter ,correct ?
If you have water with alkalinity of 95 as CaCO3 (as you do here) that really means the alkalinity is 95/50 = 1.9 mEq/L. Thats how much acid the analyst had to add to 1L of your water to get the pH to 4.4 (that's the value Ward Labs uses). If he added 1 mEq/L and stopped the pH would be lower (but not as low 4.4) and the alkalinity of the sample would be reduced (to 1.0 mEq/L = 50 ppm as CaCO3).As the alkalinity level drops it goes to more acidic level right ?
No, when you add the base malts, which typically have pH's well above desired mash pH, pH will go up and you will need a bit more acid to compensate for this.
Alkalinity is the amount of acid you must add to a water (or malt) sample to get it to reach a standard pH level (usually 4.5 in the case of water). Thus you need a pH meter (or other means of determining pH such as a colored indicator dye) and a means of dispensing acid in accurately metered amounts in order to measure alkalinity.
If you have water with alkalinity of 95 as CaCO3 (as you do here) that really means the alkalinity is 95/50 = 1.9 mEq/L. Thats how much acid the analyst had to add to 1L of your water to get the pH to 4.4 (that's the value Ward Labs uses). If he added 1 mEq/L and stopped the pH would be lower (but not as low 4.4) and the alkalinity of the sample would be reduced (to 1.0 mEq/L = 50 ppm as CaCO3).
This can be confusing to beginners who have probably been taught that pH is a measure of how acid or basic something is. That's not really the case. Acidity and alkalinity depend on the chemical composition of the solution and a pair of pH's. For example, the alkalinity of your water, between pH 8.1 and 4.4 is 1.9 mEq/L. The alkalinity of your water between pH 8.1 and mash pH (5.4) is about 90% of that.
Is my water spot on for a stout ? Just curious because it turned out fantastic without doing any additions because it was brewed before my water report.