Home canners usually boil their jars prior to filling. The stakes are higher with home-canned food, particularly low-acid foods, given the pathogens that can take hold. But not a bad idea to employ similar precautions with wort.




Which is sorta why I asked because the info I've read on pressure canning says it's not necessary.Home canners usually boil their jars prior to filling.

Home canners usually boil their jars prior to filling. The stakes are higher with home-canned food, particularly low-acid foods, given the pathogens that can take hold. But not a bad idea to employ similar precautions with wort.
View attachment 843355
I'm sure there's a good reason but I still have to ask: Why?I can turkey in quart masons















You can keep a full container solution of iodophor or starsan in a glass fermenter and that should keep the container sanitized but that gets emptied and wort is then put in it. In the case of brewing a batch, the wort gets boiled then transferred. I don't see how you are equating that to the starter, because it seems like you are saying that you don't boil the wort for the starter. If you are, you are doing the same thing. I'm not sure what point you are advocating for or advocating to drop?Several years ago, after deciding to ditch the whirly box and feeling comfortable enough with the SNS method to burn my ships and go all-in on SNS, I figured it was a good opportunity to really question everything I thought I knew about starters.
As such, I learned that storing my 1gal SNS jugs wet in a solution of iodophor worked perfectly fine for starters. I know you're supposed to pucker up and be super duper careful and super duper serious about starters, but they're nothing more than small batches of beer. If it's good enough for your fermenter, why isn't it good enough for your starter vessel? Gooses and ganders, or ganders and gooses, or something. It works, okay?
Can't say I miss the Erlenmeyer flask volcanoes.
If you successfully can your wort, it's sanitized, right? Using the SNS method, you just pour your starter wort in your jug, pitch your yeast, then shake it.You can keep a full container solution of iodophor or starsan in a glass fermenter and that should keep the container sanitized but that gets emptied and wort is then put in it. In the case of brewing a batch, the wort gets boiled then transferred. I don't see how you are equating that to the starter, because it seems like you are saying that you don't boil the wort for the starter. If you are, you are doing the same thing. I'm not sure what point you are advocating for or advocating to drop?
My process: [...]
So you don't boil the DME, although most modern day extract beer processes recommend you do, to pasteurize it.remove boiling pot from stove, set in kitchen sink, stir in 3 drops of Fermcap, then stir in DME and nutes.
- lid pot, turn on cold water
I had this happen to me, now I just use propper starter. Saves time needing to make the starter too.20+ years never happened.
View attachment 843211
-but I do know that's the last time I'll be boiling wort directly in an Erlenmeyer to sterilize. Pressure canned wort from here on out.
I wasn't sure what was going in to your starter vessel but yes if the wort was successfully canned it's sanitized. Spraying the outside of the jar would be reasonable on the pour. Hitting it with O2 is better than SNS in my opinion. I don't have an opinion on the second shot of O2.If you successfully can your wort, it's sanitized, right? Using the SNS method, you just pour your starter wort in your jug, pitch your yeast, then shake it.
That's too much work for me. I've built a lid that incorporates a stone. I pitch before I go to bed, blast it with 02, then give it a squirt when I fire up my rig the following morning.
Starter done, pitch at noon.
Honestly, I'm using Proper Pitch more and more these days because canning wort is a drag and sixteen bucks is a lot cheaper than the value that I place on my Sunday morning.
I freeze batches of starter wort at double (or even triple) gravity. Stored in sturdy 48 oz cottage cheese containers in the 2nd freezer.I do from time to time consider overbuilding and freezing.
Like you, I also don't have an opinion on the second shot of O2. I'm not sure there's a literature available on my lazy way of doing SNS, it just seems like a good idea based upon SNS principles. Most importantly, it makes me feel a bit more like I'm making a super serious starter (sorta like cracking an egg into an instant cake) and it seems to work. I'm okay with years of "seems to work."I wasn't sure what was going in to your starter vessel but yes if the wort was successfully canned it's sanitized. Spraying the outside of the jar would be reasonable on the pour. Hitting it with O2 is better than SNS in my opinion. I don't have an opinion on the second shot of O2.
My pressure canner broke so I haven't gotten to canning wort. I do from time to time consider overbuilding and freezing. A gallon isn't really a lot and a double batch split and keep one batch would be too much freezer space. Second freezer also broke, not replacing. So I use DME.
I take up half the lower freezer drawer with hops. With only two drawers, the lower is 2/3+ of the freezer, so I take up 1/3 with brewing already. My wife is fine at math but if she even estimates it, she'd think it was only 1/4. I think everyone in the house though just blanks that section out so I don't want to draw attention to a new spotI freeze batches of starter wort at double (or even triple) gravity. Stored in sturdy 48 oz cottage cheese containers in the 2nd freezer.
Before using, it gets diluted to starter gravity, re-boiled and chilled.
So you don't boil the DME, although most modern day extract beer processes recommend you do, to pasteurize it.
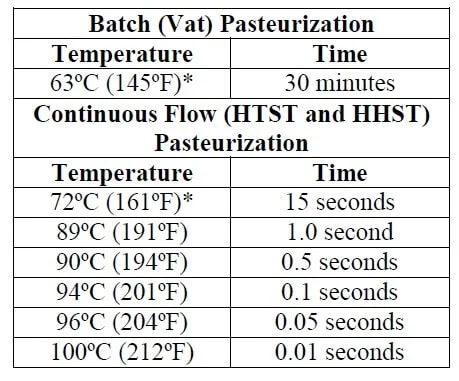
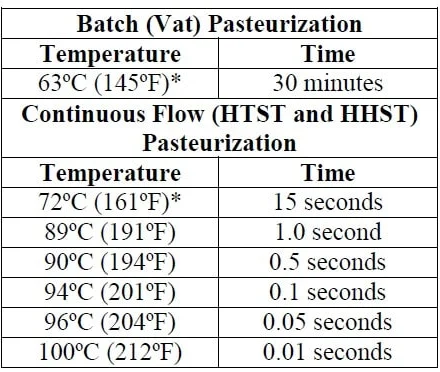
Good chart! I like how it shows multiple possibilities.Oh, but I am in fact pasteurizing the starter wort! Using High Heat Short Time process.
Pasteurization happens quite rapidly as temperature approaches boiling.
View attachment 843392
One reason why I've never had an evident infection...
Cheers!
When discussing botulism, it's important to consider both the botulism spore and botulinum toxin. If you notice the above quote, the heat-resistant endospore is active at temperatures from 40°F to 250°F (4°C to 121°C) and in conjunction with the other conditions. The toxin produced however is heat labile, (can be changed or destroyed by heat) at 176°F (80°C) for 30 minutes or 212°F (100°C) for 10 minutes (same publication). This is why low-acid foods (pH > 4.6) need to be pressure canned and high acid foods can be water bath canned. Increased pressure is needed to get the temperature to 250°F.Clostridium botulinum is the bacterium that causes botulism. Clostridium botulinum is a Gram-positive, slightly curved, motile, anaerobic, rod-shaped bacterium that produces heat-resistant endospores. These endospores, which are very resistant to a number of environmental stresses, such as heat and high acid, can become activated in anaerobic environments, low acidity (pH > 4.6), high moisture content, and in temperatures ranging from 40°F to 250°F (4°C to 121°C) (Sobel et al. 2004). In hostile environmental conditions, the heat-resistant spores enable the bacteria to survive for extended periods of time in a dormant state until conditions become more favorable.
The above touches on several conditions in regard to brewing. I am not so versed in brewing science to be authoritative about beer chemistry over time during fermentation, however beer yeast are competing microorganisms and the ph of beer is going to cross into the high acidity range in roughly 24 hours after pitching healthy and sufficient numbers of beer yeast. Very important to note is that the spore is ubiquitous (found everywhere) in nature. Nowhere along the way are we heating to 250° when brewing a batch. Even if we pressure can the starter, all bets are off once the starter liquid is cracked open unless you brew in a NASA lab, as the spore is ubiquitous. It's the drop in pH that does the trick of inhibiting the spore from producing the toxin. As far as toxin production consider carefully the conditions for which the spore can be active during your process. Boiling the wort for 10 or so minutes would destroy botulinum toxin or heating for 30 minutes at 176°F. I'm not familiar with what specific levels of other growth-limiting conditions besides heat that might also destroy the toxin. Good brewing practices help to very much limit its growth, as do good canning practices, as do proper food handling procedures. So much so that it is very rare that cases occur from food nowadays. The consequences for not doing so can readily be dying (and possibly winning a Darwin award).WHAT CAN BE DONE TO PREVENT ILLNESS?
Primary growth-limiting factors for C. botulinum include environmental temperature above 250°F (121°C) or below 39°F (4°C); high acidity (pH <4.6); low water activity (lack of available moisture); food preservatives such as nitrite, sorbic acid, phenolic antioxidants, polyphosphates, and ascorbates; a low redox potential (absence of oxygen); and competing microorganisms (Sobel et al. 2004). To be safe, the FDA 2013 Food Code recommendation is that food be kept out of the 'Danger Zone'. Thus, for safety against this pathogen and others, store food items below 41°F (5°C) and hold hot food above 135°F (57°C) (FDA 2013). Due to their low water activity, dehydrated foods and foods high in salt and/or sugar do not support growth of C. botulinum. Some strains of C. botulinum can be mesophilic, with an ideal growth temperature between 68°F–113°F (20°C–45°C), whereas others are psychotropic, with ideal growth between 38°F–60°F (3°C–20°C). Proper cooking and handling of food is important for the elimination of C. botulinum, because growth is possible at a wide range of environmental temperatures. Although C. botulinum typically will not grow in environments of pH <4.6, food proteins, such as those in soy and beef, can have a protective effect on the bacteria by providing localized areas or pockets of high pH, thus allowing for growth in high-acid foods (Wong et al. 1988).
It requires dedication to the single purpose, but my payback is minimal time heating/cooling. One must sometimes minimize kitchen use, especially when doing "that nasty smelling brewing thing", according to the kitchen proprietor.Great! But, that requires actual pot-watching.
I can leave the kitchen and come back randomly later to a boiling pot of water.
Worst thing that could happen is a slightly higher OG...
Cheers!

Yep. That’s the ‘heirloom’ canning pot I use to hot soak the jar until filling for the pressure cooker. It was SWMBO’d’s Grandmother’s. Those Graniteware pots were made to last!Home canners usually boil their jars prior to filling. The stakes are higher with home-canned food, particularly low-acid foods, given the pathogens that can take hold. But not a bad idea to employ similar precautions with wort.
View attachment 843355
Fun fact, if you dry fire graniteware for more than 3 hours, the enamel will crack and peel.Yep. That’s the ‘heirloom’ canning pot I use to hot soak the jar until filling for the pressure cooker. It was SWMBO’d’s Grandmother’s. Those Graniteware pots were made to last!