PHBalanced
Well-Known Member
I'm still curious on the original intent of this thread. Has anyone got data to go with blackened bottoms?

I probably won't bother covering the holes. I kind of like the convenience of being able to easily view the flame level without standing on my head. It looks like I may be using a little more propane, but i think I can just turn up the burner some and it won't take any longer to reach a boil. One odd thing I noticed when running this test. The rate of heat gain decreased as the water temperature increased. The rate was quite stable on the first run. I logged the time and temp at five minute intervals for both tests. I have no explanation for this difference.
This is a TOTAL guess, but maybe convective currents? Without vents, the side of the keggle stay cool, so you will have a strong heat gradient from the bottom up, allowing water to rise in the center and fall on the sides. With the vents, the sides of the keggle will heat up too, meaning the water won't want to fall down on the sides as much. You'll get a hotter outside and a cooler core in your keggle, perhaps, leading to less even heating and a longer heating time.
Again, total guess.
I'm still curious on the original intent of this thread. Has anyone got data to go with blackened bottoms?
Maybe, but the vent holes are only on the back side and span about 10". Just bases on my casual observation, there seems to be considerable convection turbulence happening while heating. I doubt that the gasses escaping through the vents actually heat up the side of the kettle much as they don't really hug the side much. Might even heat the sides less than the usual hot gasses escaping more uniformly from the bottom of the non-vented skirt. I'm guessing a this too. The change in the rate was substantial. Much more than I would have ever expected. The rate was about 4 degrees per minute and dropped to about half of that as the boiling point was approached. The tests were both run in my garage so there was no wind or breeze either.






![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)





How much time in between boils? If the first one chilled your propane tank, it could have dropped the supply pressure for the second one.
Edit: the more I think about this, the more I think this idea is wrong too....from your timestamps, it was at least a few hours in between boils...and it would take a bigassed freeze on your propane tank to drop output enough to bump up heating time by that much....
Blackening alters the emissivity of 316 only 40% while it moves aluminum 90%; big difference. Still, I'm curious to see if the difference on stainless will be measurable. I have little doubt that those replicating the experiment on aluminum will appreciate a difference.
The calculation itself is pretty straight forward to make using the Stefan-Boltzmann law but we have to make some assumptions. I assume that the emissivity of the original aluminum bottom of my boil kettle is 0.07 which is the reported literature value for aluminum. I further assume that with the black engine paint in place the emissivity is 0.9 which also seems reasonable based on literature values (http://www.infrared-thermography.com/material-1.htm). Finally we have to assume the temperature of the propane flame beneath the kettle. I'm less sure of the true average value here so I'll use the peak flame temperature to give a best case scenario. Realistically we know that the average temperature below the kettle's surface is less than this so the difference in blackened vs. silvered is less. Also, my boil kettle has an outside diameter of 13.5" which is what I'll use for my calculations.
Given all the above, the original surface experiences a radiative heat gain of 9,611 watts. When blackened the same surface experiences a radiative heat gain of 123,576 watts. My burner is rated as capable of putting out 185,000 BTU/hr which is only 54,000 watts. Clearly my assumption about the average temperature below the boil kettle is way off but you get the idea. You get drastically better heat transfer to the kettle which should give you significantly shorter rise times.
So you're saying I have a chance?
So you're saying I have a chance?
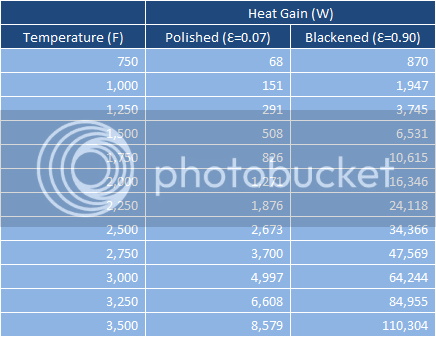
The burner output is not included anywhere in the calculations. I merely make an assumption about the average temperature of the surroundings radiating to the kettle bottom. I assume that the kettle skin temperature is 212F (which may well be too low) and then make a guess as to what the temperature below the kettle is. This lets the Stefan-Boltzman equation do its thing and then I switch the emmisivity between 0.07 and 0.9 to see with that set of assumptions how much more radiative heat transfer would occur to a blackened kettle. Below is a chart generated with various average temperatures.
Radiative Heat Transfer to Polished/Blackened Kettles

It is worth noting that it takes around 3,000,000 Joules to bring 6.25 gallons of wort and a 35 quart aluminum kettle from 160F to boiling (160 chosen as the average wort temperature after a one hour fly sparge). If you batch maybe that initial temperature is more like 170F.
Wow, that looks like a trap if I've seen one. Still, I'll put my foot in and see if I get lucky. During my test a brought five gallons of tap water from 84F to 212F which should require 5,832,830 Joules to do. I did it in 20 minutes and observed an extremely constant temperature increase which means I was putting 4,861 watts (NET) into the kettle/wort system (a Joule per second is a watt for those non-scientists following along. Joule is a unit of work, watt is a power which is the rate at which work gets done). This makes me think strongly that the average temperature below my kettle is 1,250F or lower so I'll pick 1,000F.
If we pick this temperature, blackening should net us an extra 1,800 watts. So 4,861 watts net blackened and presumably 3,065 watts net polished. This would increase the time to boil to 32 minutes as opposed to the 20 minutes I experienced. This washes with my limited experience so at least as a first approximation it feels believable.
I think this is the best thread going right now. Homebrewers are always going through great lengths and cash to increase their brewing efficiency. Also I know there are not too many direct heat breweries out there, but I could see them interested, too.
The next step if this works well is how I'm going to cure the high temp paint on my keggle. But I'm sure we can figure it out.
Don't think of it as something just to save money on propane. That's an added benefit, but I think the real value would be in time saved and having to get propane refills less often. If you think of it that way, you're saving money on car trips, too.