ChemE
Well-Known Member
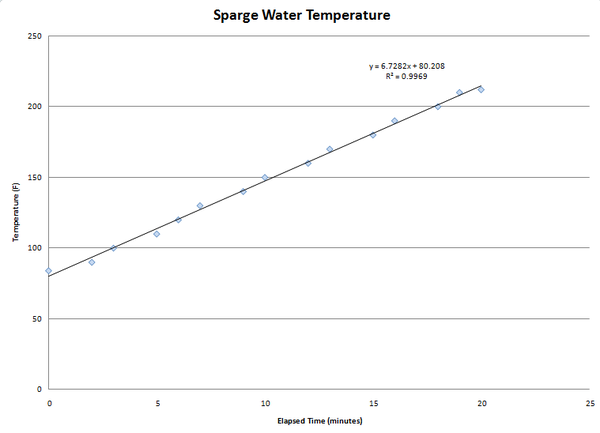
I looked around a little and didn't find any threads on this so I thought I'd share in case this is new to Homebrewers. Ultralight backpackers use this trick to get faster boils with less fuel...
Painting the bottom surface of your boil kettle with flat back engine paint will drastically increase the emissivity of the surface which allows it to absorb heat much more readily as compared to a surface with a very low emissivity. A little painters tape and some newspaper makes the finished product look nice and neat.
Freshly blackened pot bottoms

I really should polish up the aluminum sides of the pots to further drop their emissivity so that less energy is radiated to my garage but its such a hassle I'm not sure I'm up to the task. Anyone have a good/fast/easy way of getting a nice polish on such a large aluminum surface?
Painting the bottom surface of your boil kettle with flat back engine paint will drastically increase the emissivity of the surface which allows it to absorb heat much more readily as compared to a surface with a very low emissivity. A little painters tape and some newspaper makes the finished product look nice and neat.
Freshly blackened pot bottoms

I really should polish up the aluminum sides of the pots to further drop their emissivity so that less energy is radiated to my garage but its such a hassle I'm not sure I'm up to the task. Anyone have a good/fast/easy way of getting a nice polish on such a large aluminum surface?