Martin said that although the brown malt is roasted that I should treat as a base malt. Treating it as Roasted lowered the predicted ph to 4.30. Note that the 5.18 (base malt) and the 4.30 (Roasted) were with no salts.
Martin must have some reason for telling you that brown malt is more like a base malt of similar color but that flies in the face of common sense and if it is indeed like base malt then there is no way the predicted pH would be as low as you have been finding. In fact you should find, as you intuited, that acid would be required rather than alkali. If you are getting pH's as low 5.18 with two base malts entered with alkalinity as high as yours that suggests error in data entry.
Are we assuming that the low prediction is due to the way that the Brown Malt is being considered? I could input any base grain with a 65L color and still get the same results, so I'm wondering what we're learning with respect to this recipe.
You have hit upon the basic shortcoming of this (the spread sheets') method of mash pH prediction. It assumes that a malt's titration 'curve' (they assume it is linear - as we shall see in a moment it isn't) is predictable from its color or type. In fact there is quite a bit of variability in the parameters of malts of similar color and/or type.
This maybe a stupid idea, but has anyone thought of asking Martin or Kai why the predictions are so low for this grist bill?
The answer is that the prediction are as low as they are because of the data you are feeding the programs. What you are getting does not seem to make sense in terms of what we expect from these programs and so we suspect you may be entering something in error.
I want to make sure I have this right testing as you suggested with the brown malt. The 1st test I would record the pH.
Let's assume that the malt has identical properties to the 80L Briess malt that I measured. In your first measurement you mix a kg of it with 2L of mash temperature DI water (you wouldn't need to use nearly this much but let's keep the math easy here), let it sit for 20 minutes, pull off a sample of the liquid, let it cool to room temperature and measure the pH. It would be 4.77.
The 2nd test I would add an amount of bicarbonate estimated to shift the pH up say by .1, measure the actual shift.
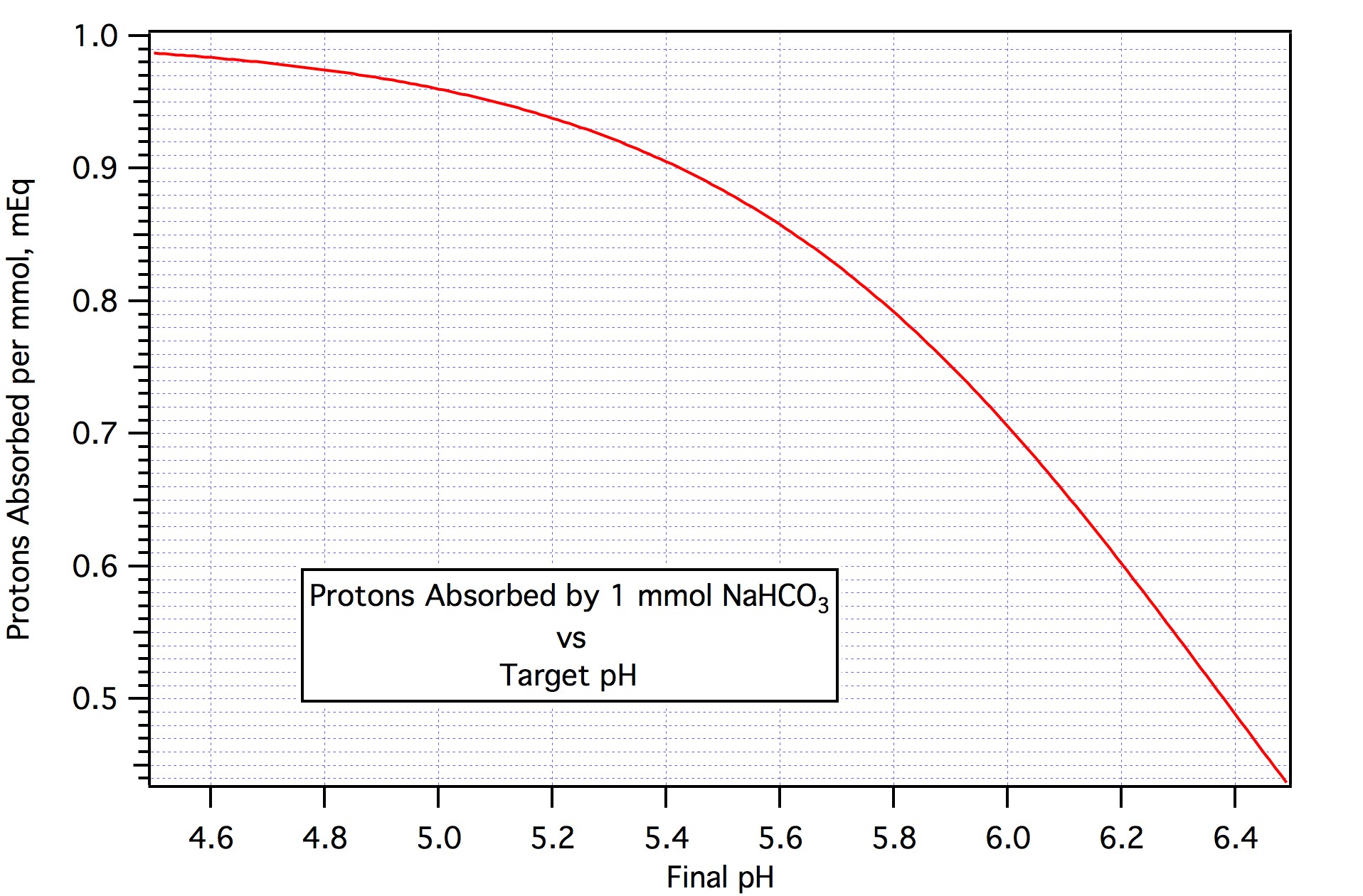
For the second test you note that with this low DI mash pH that the malt is pretty acidic and so decide to add a gram NaHCO3 to another 2 L of DI water and mash another kg of the malt using the same procedure. Again you don't need that much malt or water in reality. This time you get a pH of 4.90. A gram of NaHCO3 is 1000/84 = 11.9048 mmol and by consulting the curve in the earlier post you see that at pH 4.90 each mmol of bicarbonate absorbs 0.967948 mEq protons (you can't read that accurately from the curve, of course, thus I am using calculated values) so that you have absorbed 11.9048*0.967948 = 11.5232 mEq protons. You would then calculate (11.5232)/(4.77 -4.90) = -88.64 and realize that this malt has, ostensibly, more buffering capacity than the 'average' malt.
Your next step would be to plot the measurements you have made on a graph. You don't have to do this because you can do the extrapolation to target pH without it. The model is mEq/kg = -88.64*(pH - 4.77). The bicarbonate estimate for pH 5.4 is simply -88.64*(5.4 - 4.77) = -55.8432 mEq/kg. The - sign simply indicates that this is the quantity of protons that must be absorbed. The bicarbonate required is then 88.64*(5.4 - 4.77)/0.9052 = 61.6916 mmol/kg as each mmol of bicarb supplies 0.9062 mEq of proton absorbing capacity at pH 5.4. The plot will certainly aid in your understanding. It will look like this:
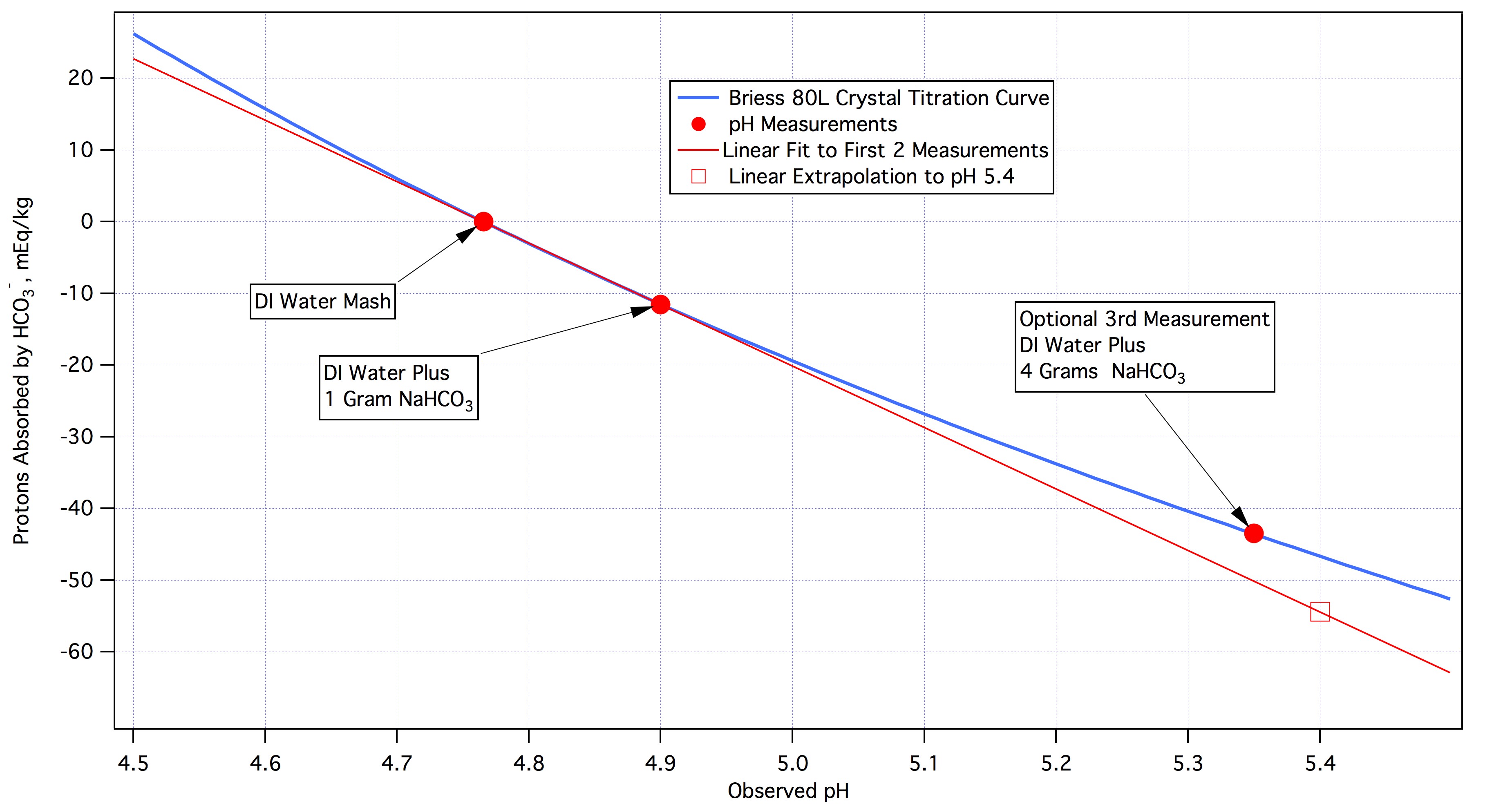
The solid dots at the left are the results of the two measurements and the thin red line is and extension of the line connecting the dots. The open rectangle is the point on that extrapolating line at pH 5.4 which is a reasonable target pH. The heavy blue line is the actual titration curve from this malt which, as is plain, is not linear. Whereas the linear extrapolation would cause you to conclude that you need to absorb about 55 mEq protons per kg of this malt in fact you need to absorb only about 47. This illustrates another problem with the basic spreadsheets. They must assume linearity as without it they would have to use recursive techniques to get pH estimates. The problem rears its head when DI mash pH is distant from the target pH. Fortunately this is only the case with specialty malts which tend to be used in smaller quantities than the base malts whose DI pH's tend to be within a couple of tenths of target pH.
I am still going to need to make another test with both grains and salts.
Clearly in the case of this malt (and we are not suggesting that it is necessarily a good example to use when discussing Brown Malt) another measurement is warranted. On the plot we show that a 3rd measurement with 4 grams of NaHCO3 would get us closer to target pH. With that third measurement in hand we can either
1)Throw away the second measurement and do a linear extrapolation using the first and third measurements. This gets us a required absorption estimate which is within half a mEq/kg of the actual
2)Fit a quadratic to the three measurements thus getting a model for this malt that includes its non linearity.
At this point you are probably thinking that this is a lot of work and most people aren't interested in understanding the underlying science to the point where they can practically use information like that which we have illustrated here. Even if you get detailed data on a library of malts there is no place to take it unless you put together your own spreadsheet (which is actually very easy to do if you understand the science the hard part of which is the bicarbonate chemistry).
In your case I think I'd at least do the DI test mash on the Brown Malt to see if it is unusually acidic. If it turns out to be then I would doubtless make additional measurements. If not then a test mash or two with your actual brewing water would be the way I'd go.
What I was doing for water chemistry in the past was based on a recommendation of a local brewer here who had a nano-brewery and had the same water that I have. For malty beers add 1/8 to 1/4 tsp CaCl/5gals. For hoppy beers add 1/8 to 1/4 tsp gypsum/5 gals.
I, in essence, do exactly that but use RO water as the base.




![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)


























































