FredTheNuke
Well-Known Member
I'm close to attempting to wake a few up from October 2011 soon here...

So I'm still curious how to know if my slants are good or have gone bad.. right now they kind of look like a white cream has been spread on them.. almost like icing on top of the agar. But a poster a few above here said his were fuzzy :-/


Wow, what a thread!
Many thanks to the OP for starting this.
I'm slowly accumulating all my equipment to start doing this and I have a couple of questions:
1) Is 96.6% Bioethanol OK to use (diluted to 70% I assume) for sanitizing and cleaning?
2) I'm especially interested in slanting/stabbing multi-bug yeasts/bacteria such as theRoeselare, Brett. Lambicus, Lacto and Pedio. Will slants/stabs work or do I need to do anything else special for the sour bugs?
The media recommended for use and found to be the most beneficial to this study were MYPG, WLN, and CuSO4. Additionally it was found that storing cultures at room temperature in a nutrient rich liquid substrate maintained the integrity of cells longer and can be used for repeated propagations.
Andrikos,
I'm not sure what answer to give you concerning "Multi-Bug" storing. It appears that different conditions would benefit different types of bugs/yeast.
From Chad Yakobson stated this in his dissertation:
It appears that his advise from Brett is the opposite of what I would do for brewer's yeast.
Look here for more info:
http://www.brettanomycesproject.com/dissertation/conclusion/







![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)





Andrikos,
I would recommend that you listen to this podcast as well. It's long. It's not all about Chad and the Crooked Stave brewery. You may want to just fast forward to the interview. There is a lot of gold in this. He talks about brewing with Brett. Storing Brett. And recipe ideas with the bug as well. He also talks about how he culturing different strains other than just what is available from the commercial labs.
http://thebrewingnetwork.com/shows/866
Jason
I'm close to attempting to wake a few up from October 2011 soon here...
I've always followed the ratio in the OP. 400ml water, 35g DME, 2.5g Agar. Today I went to make up some more plates, as I plan on trying to harvest from the can's of Heady Topper I just got my hands on. I've always been annoyed by the break material that ends up in my slants/plates so I decided to try something a bit different.
I took 400ml worth of sterile wort (extra runnings from prior batches that I collected in mason jars & then sterilized in my pressure cooker) and added 2.5g of Agar and some food coloring, let it bloom for 20 min and then brought it up to 180F. I then poured my plates and they're in the pressure cooker right now. We'll see if then end result is any better..
My only annoyance I'd love to solve, is the little bit of liquid the ends up in the bottom of the slants.
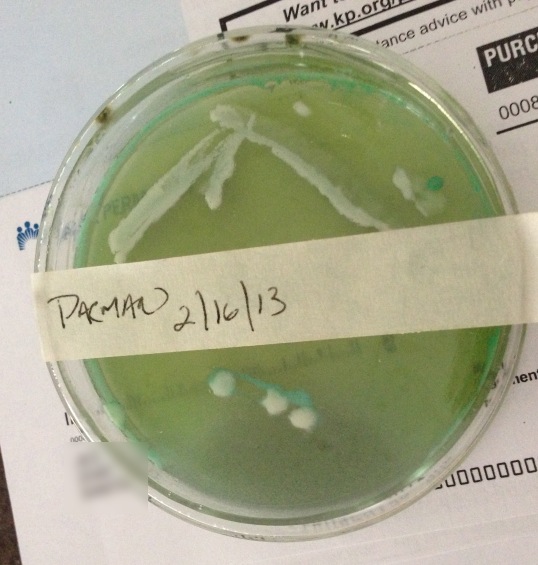
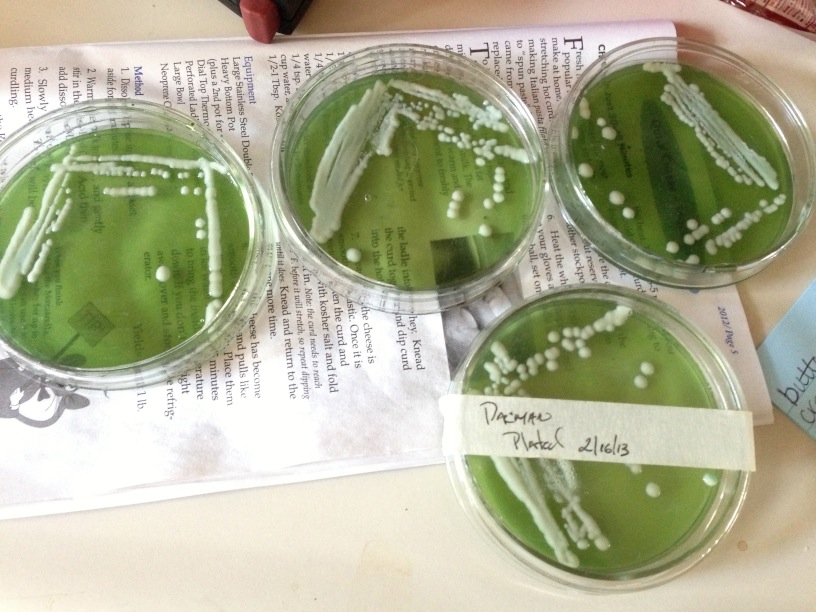
Ive been thinking of doing some yeast slants, I just have one newb question...
What does the actual 45* surface angle achieve? is it just for a larger surface or what?
A fully covered bottom will allow co2 to build up under the media and push the media to the top of the vial.
diS said:How important is to incubate slants (or plates in my case) at 70-80F?
I can't use my ferm. chamber to control temperature since there is amber ale fermenting at the moment, and it is 60-70 in my home where I keep my plates..
I assume it is not that big deal and it will only slow things, am I right or should I organize some sort of heating?
I want to toss out there I do the oil immersion technique in YEAST now (started a couple weeks ago). I emailed white labs about it. I asked if the mineral oil used for butcher blocks was fine. And for the heck of it I inquired if 100% olive oil was ok. They never heard of using olive oil so did not recommend it. As far as the mineral oil, they just said mineral oil without being specific about 99.9% or 100%. I couldn't find any 100% mineral oil, but did find some 99.9% mineral oil for consumption (for constipation or something or other) at a rite aid or walgreens. The other .1% is vitamin E. They recommend it be sterilized before use, of course.
I am just in the stage of accumulating equipment to start making slants but I have been contemplating adding mineral oil to extend the "shelf life" of the slants. One thing I have been wondering about is sterilizing the tool (dropper, pipette, etc.) that you use to add the mineral oil. Probably over-thinking things but how do you manage this?
I am just in the stage of accumulating equipment to start making slants but I have been contemplating adding mineral oil to extend the "shelf life" of the slants. One thing I have been wondering about is sterilizing the tool (dropper, pipette, etc.) that you use to add the mineral oil. Probably over-thinking things but how do you manage this?
very glad that I came across this thread. i have been wanting to culture yeast somehow so I dont have to keep buying fresh yeast for every brew and this seems like the best method. but I do have a question. Say i make 5 slants from one fresh yeast culture. If I want healthy first generation yeast for ever brew will i have to buy fresh yeast after these slants are used up? Or once i make a starter from one of these slants can I then use that starter to inoculate 5 more slants? and essentially never run out of this yeast strain? Or would a slant made from this starter not be considered a first generation yeast?