Wow..... the following is very telling.......
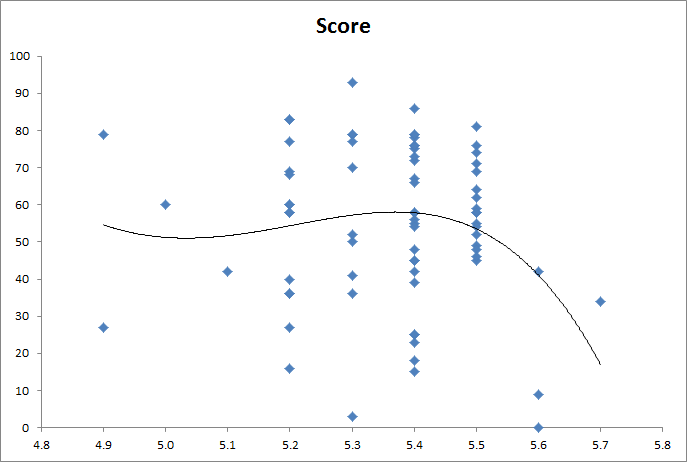
I always keep track of various parameters for every batch. One parameter is mash pH, typically measured after about the first 10-15 minutes in. These are reported at room temp, by the way. Another parameter is a normalized quality score on a 0-100 scale. I won't get into how that's determined at the moment but suffice it to say that I try to evenly distribute as many scores from 0-50 as I do from 51-100. Following is a plot of pH vs. quality score for my last, I dunno, ~100 batches.
View attachment 639240
What I am able to conclude from this new graph, which I've never done until now, is that if I mash at 5.5 @room temp (which would be about 5.25 at mash temp ~150 F), then I am extremely likely to end up with a high quality beer.
How do you like that?! I sure as hell know what to aim for now. In fact I'll even go higher to 5.6, since I only have 3 data points there currently. I imagine that part of the curve will shift upwards with more data points, but, maybe not. Maybe there truly is a ceiling, at 5.5 for me and my process, beyond which the quality goes bye-bye.
P.S. And yes, I've always rounded to the nearest 0.1 pH unit. Good enough for me.




























![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)