Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
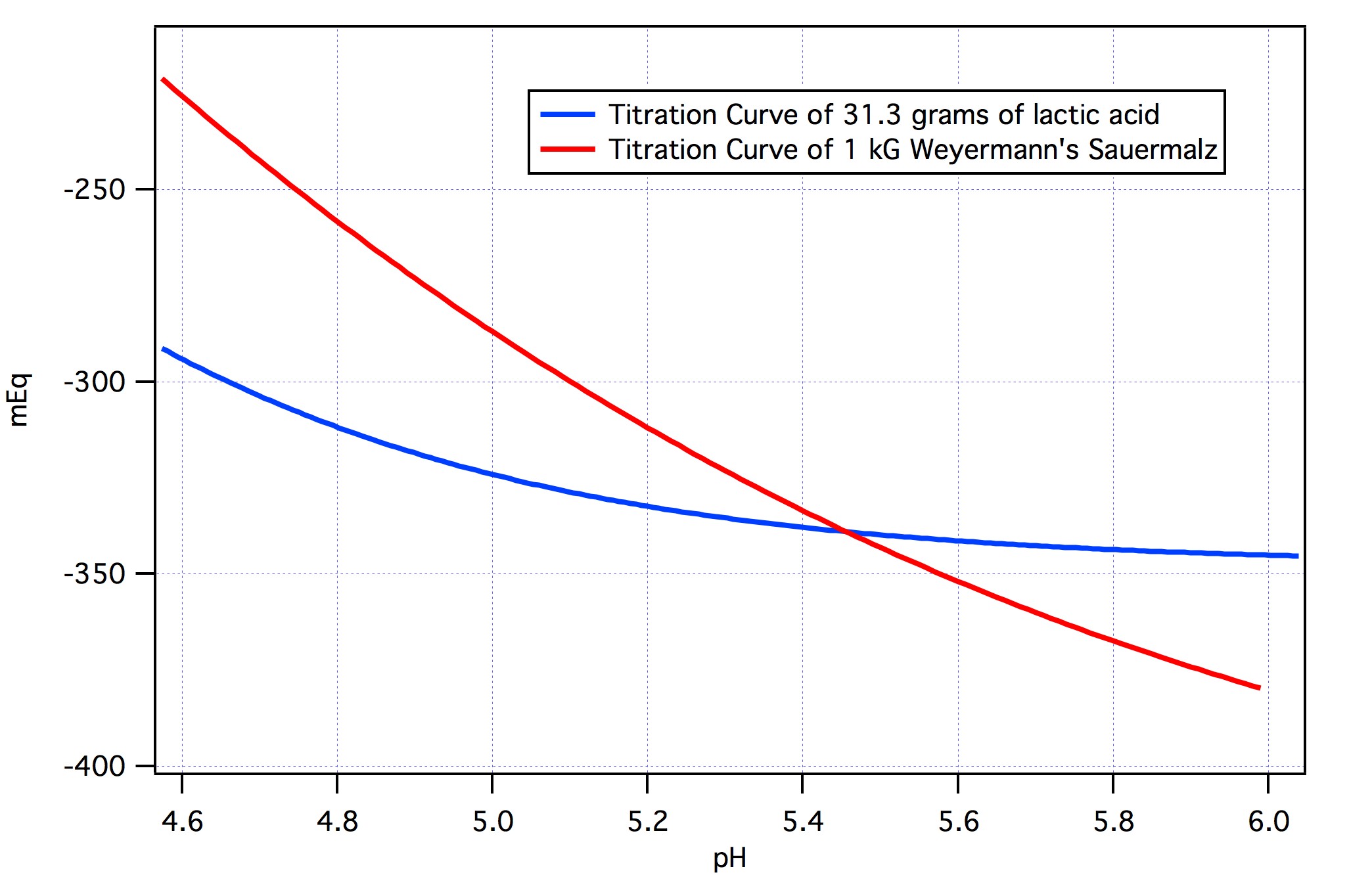
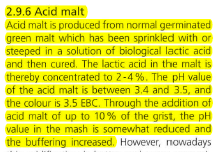
I'm planning to brew a Dry Irish Stout, and I'd like to substitute 12 Oz. of Acid Malt for 12 Oz. of the recipes base malt in order to induce some old fashioned Guinness like twang (lacking better terminology here). But I would also want to fully neutralize this 12 Oz. of Acid Malt via the use of Calcium Hydroxide (Pickling Lime). I've preliminary worked out that roughly 4.3 grams of pickling lime should be sufficient to neutralize 12 Oz. of a nominally average strength Acid Malt. Is ~4.3 g. of Ca(OH)2 the correct amount to add, or should I plan to add some other quantity of pickling lime?





































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)