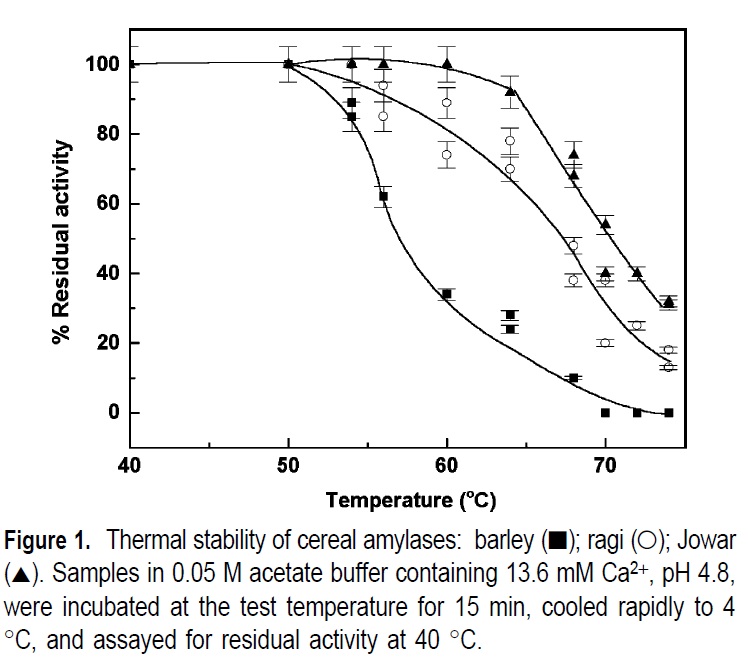
All the brewing sources mention that enzymes are easily deactivated or denatured by temperatures much outside their range. Ie 131 to 150F. (http://www.howtobrew.com/section3/chapter14-5.html)
So what happens when one adds heat to a mash via a flame under the kettle, an electric heating element or a HERMs coil ? If the mash is at, say, 140F and you want to heat it up to 150F, the heat source needs to be quite a bit higher than 150F. 160F ? 180 F ? 200F ?
When the amylase touches the heat source, won't it be ruined ?
Is the amylase dissolved in the liquid part of the mash or is it stuck to the solids or both ?
Are some heat sources better than others in this respect, in that they have more area and run on a lower temperature differential ?
So what happens when one adds heat to a mash via a flame under the kettle, an electric heating element or a HERMs coil ? If the mash is at, say, 140F and you want to heat it up to 150F, the heat source needs to be quite a bit higher than 150F. 160F ? 180 F ? 200F ?
When the amylase touches the heat source, won't it be ruined ?
Is the amylase dissolved in the liquid part of the mash or is it stuck to the solids or both ?
Are some heat sources better than others in this respect, in that they have more area and run on a lower temperature differential ?




































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)