Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
What if charts like these are wrong (or rather, are correct only for certain idealized temperatures and/or salinities that have zero to do with brewing)?


What if the reality was more akin to being all over the board such as for this chart which adds the impact of a single temperature change from 5 C. to 25 C., plus a single salinity % change.
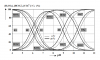
Then imagine what the reality might be like when mash temperature is bumped to ~67 C. and salt (whereas minerals of all kinds can be referred to as salts, and not just NaCl) levels are magnitude multiples of your mineral additions due to the high inherent salt content of the malts/grains themselves. Who knows what the carbonate species reality might look like then, but one certainty is that any software based upon either of the first two charts seen above will be massively wrong when applied to a mash, or even to an attempt to target a pH for ones sparge water.


What if the reality was more akin to being all over the board such as for this chart which adds the impact of a single temperature change from 5 C. to 25 C., plus a single salinity % change.

Then imagine what the reality might be like when mash temperature is bumped to ~67 C. and salt (whereas minerals of all kinds can be referred to as salts, and not just NaCl) levels are magnitude multiples of your mineral additions due to the high inherent salt content of the malts/grains themselves. Who knows what the carbonate species reality might look like then, but one certainty is that any software based upon either of the first two charts seen above will be massively wrong when applied to a mash, or even to an attempt to target a pH for ones sparge water.


